
���� ��1����ˮ��Һ�������״̬�µ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ�
��2������ͭ��ǿ����ʣ���ˮ��Һ����ȫ��������ͭ���Ӻ���������ӣ�
��3��ʵ��������480ml������ƿ��Ӧѡ��500ml����ƿ������Һ���ݴ˼�����Ҫ����ͭ��������������ˮ���ó�500ml��Һ��
��4���Ȼ���ˮ��õ������������壻
��5������ͭ������������Ӧ�������ᱵ������������ͭ������
��6����ȡ��ˮ�еĵ�IJ�������ȡ����Һ������
��� �⣺��1����ˮ��Һ�������״̬�µ���Ļ������ǵ���ʣ���ˮ��Һ�������״̬�¶�������Ļ������Ƿǵ���ʣ����⼸���������ڵ���ʵ��Т٢ݢޢᣬ���ڷǵ���ʵ��Тۢܣ��ʴ�Ϊ���٢ݢޢ�ۢܣ�
��2������ͭ��ǿ����ʣ���ˮ��Һ����ȫ��������ͭ���Ӻ���������ӣ����뷽��ʽΪCuSO4=Cu2++SO42-���ʴ�Ϊ��CuSO4=Cu2++SO42-��
��3��ʵ����Ҫ����480mL0.1mol/L������ͭ��Һ��ʵ��������480ml������ƿ��Ӧѡ��500ml����ƿ������Һ����Ҫ��ȡCuSO4•5H2O����=0.5L��0.1mol/L��250g/mol=12.5g������ˮ���500ml��Һ��
�ʴ�Ϊ��12.5��500��
��4���Ȼ���ˮ��õ������������壬���ӷ���ʽΪFe3++3 H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3 H+��
�ʴ�Ϊ��Fe3++3 H2O$\frac{\underline{\;\;��\;\;}}{\;}$Fe��OH��3�����壩+3 H+��
��5������ͭ������������Ӧ�������ᱵ������������ͭ���������ӷ���ʽΪCu2++Ba2++SO42-+2OH-=Cu��OH��2��+BaSO4����
�ʴ�Ϊ��Cu2++Ba2++SO42-+2OH-=Cu��OH��2��+BaSO4����
��6�����õ��ڲ�ͬ�ܼ����ܽ�ȵIJ����Բ�����ȡ��������ӵ�ˮ����ȡ������Ȼ����÷�Һ�������룬�����е���л��ܼ���������ķ���������⣬������ȡ��ˮ�еĵ�IJ�������ȡ����Һ������
�ʴ�Ϊ����ȡ����Һ������
���� ���⿼����ۺϣ��漰���ʷ�����ᴿ�����ӷ���ʽ����д�����ʵ���Ũ�ȼ����֪ʶ�㣬Ϊ��Ƶ���㣬��ȷ�������ʡ����ӷ���ʽ��д�������ʵ����йؼ����֪ʶ�㼴�ɽ��ע�⣨5������©������������ͭ�����ӷ�Ӧ��Ϊ�״��㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH | B�� | HCl | C�� | NaCl | D�� | K2SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
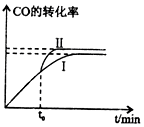 Ϊ�˼���úȼ�նԴ�����ɵ���Ⱦ��ú��������Һ���Ǹ�Ч���������ú̿����Ҫ;����������CO2������ŷ�Ҳ���������ٵ��ش���⣮
Ϊ�˼���úȼ�նԴ�����ɵ���Ⱦ��ú��������Һ���Ǹ�Ч���������ú̿����Ҫ;����������CO2������ŷ�Ҳ���������ٵ��ش���⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na��O | B�� | Na+��O2- | C�� | Mg��Na | D�� | ?����2+��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10��1 | B�� | 100��1 | C�� | 1��100 | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | m=-26.5��2c=a-b | B�� | m=-53��c2=$\frac{a}{b}$ | C�� | m=-26.5��c2=$\frac{a}{b}$ | D�� | m=-53��2c=a-b |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com