
��18�֣��ۺ��������ֳƾ�������ѧʽΪ[Fe2(OH)n(SO4)3��0.5n]m�� �㷺������ˮ������ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ��������̷�(FeSO4��7H2O)���������£�

��1����֤����W���պ���������庬��SO2�ķ����ǣ�
___________________________________________________________________��
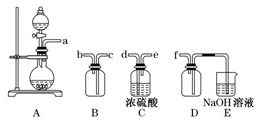
��2��ʵ�����Ʊ����ռ������SO2�������������¡�װ��A����SO2���������������Ӹ������ӿڣ�˳��Ϊa��____��____��____��____��f��
װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
װ��D��������_____________��װ��E��NaOH��Һ��������__________________��

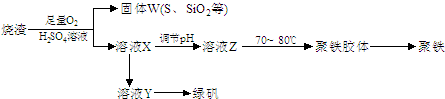
��3���Ʊ��̷�ʱ������ҺX�м������__________����ַ�Ӧ�����˲����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷�����������IJ���������_______________________��
��4�����ⶨ��ҺY��Fe2+��Ũ�ȣ���Ҫ������ƿ����KMnO4����Һ������ʱ����Ӧ_____________________��ֱ��_____________________����KMnO4����Һ�ζ�ʱӦѡ��________�ζ���(���ʽ����ʽ��)��
��5����ҺZ��pHӰ�������������������������ҺZ��pHƫС�������¾�����������������______(�ƫ����ƫС������Ӱ�족)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣��ۺ��������ֳƾ�������ѧʽΪ���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
��1����֤����W���պ���������庬��SO2 �ķ�����___��
��2���Ʊ��̷�ʱ������ҺX�м������___����ַ�Ӧ��_____�����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷���
��3����ҺZ��pHӰ�����������������������pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ____������ҺZ��pHƫС�������¾�����������������ƫ_____��
��4���Ŵ����̷����տ����̷��ͣ�Ҳ����ˮ�������ᣩ�ͺ�ɫ���ϣ�Fe2O3������д���йصĻ�ѧ����ʽ��
��
��5���̷����������·���������ɫ���ϣ�Fe2O3��,�������������ǣ���5560kg�̷���Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���16680 kg �̷���560 kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����____________________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ����һ�и��������ο��Ի�ѧ�Ծ����������� ���ͣ������
�ۺ��������ֳƾ���, ��ѧʽΪ[Fe(OH)(SO4)]m, �㷺������ˮ������ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ��������̷�(FeSO4��7H2O )�������£�
��֤����W���պ���������庬��SO2�ķ�����
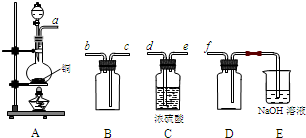
(2)ʵ�����Ʊ����ռ������SO2, �����������¡�װ��A����SO2, �������������Ӹ������ӿ�,˳��Ϊa��___ ��___ ��___ ��___f��װ��D�������� , װ��E��NaOH��Һ�������� �� 
��3���Ʊ��̷�ʱ������ҺX�м������_____����ַ�Ӧ��_______�����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷���
(4)��ҺZ��pHӰ�����������������������pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ ������ҺZ��pHƫС�������¾�����������������ƫ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ���������ο��Ի�ѧ�Ծ��������棩 ���ͣ������
�ۺ��������ֳƾ���, ��ѧʽΪ[Fe(OH)(SO4)]m, �㷺������ˮ������ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ��������̷�(FeSO4��7H2O )�������£�

��֤����W���պ���������庬��SO2�ķ�����
(2)ʵ�����Ʊ����ռ������SO2, �����������¡�װ��A����SO2, �������������Ӹ������ӿ�,˳��Ϊa��___ ��___ ��___ ��___f��װ��D�������� , װ��E��NaOH��Һ�������� ��

��3���Ʊ��̷�ʱ������ҺX�м������_____����ַ�Ӧ��_______�����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷���
(4)��ҺZ��pHӰ�����������������������pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ ������ҺZ��pHƫС�������¾�����������������ƫ_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com