
��ϩ�����������干a mol����b mol������������һ�ܱ������У���ȼ
���ַ�Ӧ����ϩ������ȫ�������꣬�õ�CO��CO2�Ļ�������45gˮ������
�� ��a =1ʱ����ϩ����������ʵ���֮��n (C2H4)��n (C2H6)= ��
�� ��a =1���ҷ�Ӧ��CO��CO2�����������ʵ���Ϊ��Ӧǰ������2/3ʱ����
b= ���õ���CO��CO2�����ʵ���֮��n (CO)��n (CO2)= ��
�� a��ȡֵ��Χ�� ��
�� b��ȡֵ��Χ�� ��
�� 1��1 �� 3��1��3 �� 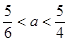 ��
�� 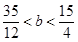
��������
�����������1������ϩ����������ʵ����ֱ���x��y����x��y��1��2x��3y��45��18��2.5�����x��y��0.5��������ϩ����������ʵ���֮��n (C2H4)��n (C2H6)��1��1��
��2����CO��CO2�����ʵ����ֱ���x��y�������̼ԭ���غ��֪x��y��2��������ԭ���غ��֪��x��2y��2.5����2���Ƿ�Ӧǰ���������ʵ�����������x��y����x��2y��2.5����3�����x��0.5mol��y��1.5mol����õ���CO��CO2�����ʵ���֮��n (CO)��n (CO2)��1��3������b����x��2y��2.5����2��3mol��
��3������ˮ��2.5mol���������ȫ������ϩ���ɵģ���a��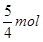 �����ȫ�����������ɵģ���a��
�����ȫ�����������ɵģ���a��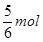 �����a��ȡֵ��Χ��
�����a��ȡֵ��Χ��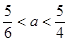 ��
��
��4�����ݣ�3����֪�����ȫ������ϩ���ɵģ���a��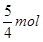 ������Ҫ���������ʵ�����
������Ҫ���������ʵ�����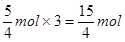 �����ȫ�����������ɵģ���a��
�����ȫ�����������ɵģ���a��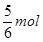 ������Ҫ���������ʵ�����
������Ҫ���������ʵ�����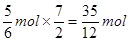 �����b��ȡֵ��Χ��
�����b��ȡֵ��Χ��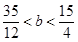 ��
��
���㣺������ȼ�յ��йؼ���
�������������е��Ѷȵ����⣬���������ӱ���������С�����Ĺؼ��ǽ�������ѧ�еļ�ֵ��������⣬��Ҫѧ����ƽʱ��ѵ����ע����ۺ��ܽᡣ


 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2 |
| 3 |
| 5 |
| 6 |
| 5 |
| 4 |
| 5 |
| 6 |
| 5 |
| 4 |
| 35 |
| 12 |
| 15 |
| 4 |
| 35 |
| 12 |
| 15 |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��a=1ʱ����ϩ����������ʵ���֮�� n(C2H4)��n(C2H6)=_______________��
(2)��a=1���ҷ�Ӧ��CO��CO2�����������ʵ���֮��Ϊ��Ӧǰ������2/3ʱ����b=_____________���õ���CO��CO2�����ʵ���֮��Ϊn(CO)��n(CO2)= _______________��
(3)a��ȡֵ��Χ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ�����и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ�������
��8�֣���ϩ�����������干a mol����b mol������������һ�ܱ������У���ȼ���ַ�Ӧ����ϩ������ȫ�������꣬�õ�CO��CO2�Ļ�������45gˮ������
��1����a=1ʱ����ϩ����������ʵ���֮��n��C2H4����n��C2H6��= ��
��2�� ��a=1���ҷ�Ӧ��CO��CO2�����������ʵ���Ϊ��Ӧǰ������ ʱ����b=
�� �õ���CO��CO2�����ʵ���֮��n��CO����n��CO2��=
��
ʱ����b=
�� �õ���CO��CO2�����ʵ���֮��n��CO����n��CO2��=
��
��3��a��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com