
| A�� | NaOHֻ��NH4HSO4��Ӧ | |
| B�� | ��NH4��2SO4��ˮ�еĵ��뷽��ʽ����NH4��2SO4=NH4++SO42- | |
| C�� | NH4HSO4�����ʵ�����0.04 mol | |
| D�� | ��NH4��2SO4��NH4HSO4���ʵ���֮����1.87��1 |
���� ����������ȫΪ��NH4��2SO4ʱ����NaOH������С��7.24g��NH4��2SO4�����ʵ���Ϊ$\frac{7.24g}{132g/mol}$��0.0548mol�������������Ƶ����ʵ���Ϊ0.0548mol��2=0.1096mol��0.1mol��˵���������Ƶ����ʵ������㣬
��NH4��2SO4��NH4HSO4�������Ʒ�м�������������Һ�����뷴ӦNH4HSO4��Ȼ���루NH4��2SO4��Ӧ���ɰ�������״�������ɰ��������ʵ���Ϊ��$\frac{1792��1{0}^{-3}L}{22.4L/mol}$=0.08mol��
���������ӷ�Ӧ����NaOH�����ʵ���Ϊ0.1mol-0.08mol=0.02mol����NH4HSO4�����ʵ���Ϊ0.02mol��
���ԣ�NH4��2SO4������Ϊ7.24g-115g/mol��0.02mol=4.94g�������ʵ���Ϊ$\frac{4.94g}{132g/mol}$��0.0374mol���Դ������
��� �⣺A��������������֪����NaOH����NH4HSO4��Ӧ�����루NH4��2SO4��Ӧ����A����
B����NH4��2SO4��ˮ�еĵ��뷽��ʽΪ��NH4��2SO4=2NH4++SO42-����ѭ����غ㣬��B����
C��NH4HSO4�����ʵ�����0.02 mol����C����
D����NH4��2SO4��NH4HSO4�����ʵ�����Ϊ0.0375mol��0.02mol=1.87��1����D��ȷ��
��ѡD��
���� ���⿼������ļ��㣬Ϊ��Ƶ���㣬���ռ��Է��жϷ����ķ�ӦΪ���Ĺؼ������ط�������������Ŀ��飬ע�ⷴӦ���Ⱥ�˳����Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Һ | B�� | ���� | C�� | �ܽ� | D�� | �����ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
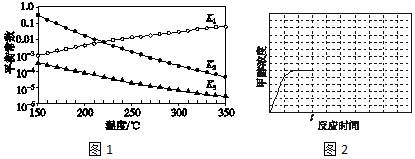
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Y��ת����Ϊ60% | B�� | ��Ӧ����v��Y��=0.3 mol/��L•min�� | ||
| C�� | a��ֵΪ2 | D�� | ƽ��ʱX��Ũ��Ϊ0.2 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ����� | |
| B�� | ����ȡ�ķ�������ú�ͺ����� | |
| C�� | ���ܽ⡢���˵ķ�������KNO3��NaCl����Ļ���� | |
| D�� | ��ij��Һ���ȼ�BaCl2��Һ�����а�ɫ�������ټ�ϡ���ᣬ�������ܽ⣬�ɼ������к���SO${\;}_{4}^{2-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
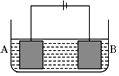 ����ͼ��ʾ��װ�ý��е��ʵ�飮A����ͭ���Ͻ�B��Ϊ��ͭ���������ҺΪ����ͭ��Һ����������ͨ��һ��ʱ���A��ǡ��ȫ���ܽ⣬��ʱB����������3.2g����Һ��������0.05g������֪�����ԣ�Cu2+��Ni2+����A�Ͻ���ͭ����ԭ�Ӹ�����Ϊ��������
����ͼ��ʾ��װ�ý��е��ʵ�飮A����ͭ���Ͻ�B��Ϊ��ͭ���������ҺΪ����ͭ��Һ����������ͨ��һ��ʱ���A��ǡ��ȫ���ܽ⣬��ʱB����������3.2g����Һ��������0.05g������֪�����ԣ�Cu2+��Ni2+����A�Ͻ���ͭ����ԭ�Ӹ�����Ϊ��������| A�� | 4��1 | B�� | 3��1 | C�� | 2��1 | D�� | 1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�Ӻ���ԭ�� | B�� | ͭԭ�Ӻͺ�ԭ�� | C�� | ̼ԭ�Ӻ���ԭ�� | D�� | ��ԭ�Ӻ�̼ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
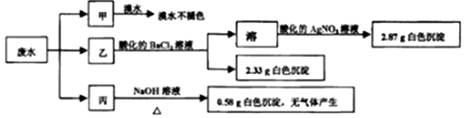
| A�� | ��ˮ���ܺ���Na+��K+��Fe3+ | |
| B�� | ���ܴ��ڵ���������ɫ��Ӧ��һ������ȷ�� | |
| C�� | ��ˮһ������Cl-��SO42-��Mg2+����c��Cl-��=0.2mol•L-1 | |
| D�� | ��ˮһ������ SO32-��Cl-��NH4+��Na+��K+��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | A | B | C | D |
| װ�ú��Լ� | 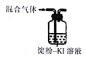 | 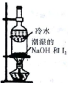 | 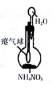 |  |
| ʵ��Ŀ�� | ����NO2�л��������� | ���볱ʪ��NaOH��I2 | ��֤NH4NO3�ܽ����� | �Ʊ����ռ�SO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com