
��10�֣��������Ļ�������������������������Ҫ����;��
(1)�ۺ�������(���PFS)�Ļ�ѧʽΪ [ Fe (OH)n(S04) (3-n) / 2 ]m ���ִ�DZˮ���������г�����PFS��ˮ�����γ���״��������ؽ������ӡ���PFS������ԭ��δ�ɶԵ�����Ϊ__ ����
(2)������������K4[Fe(CN)6]��������Ӱ�����û������д��ڵĻ�ѧ��������_____ ____ ��
��3��CNһ��̼ԭ�ӵ��ӻ���������� _______ ��д��һ����CNһ��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ_____ ___��
(4)���Ȼ���������Ϊ���壬�۵�304�棬�е�3160C ��3000C���Ͽ�������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ______ ���塣
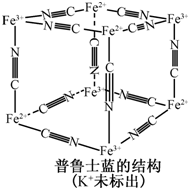
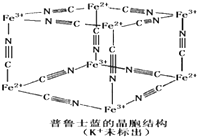
(5)��³ʿ����һ������������Ⱦ�ϣ����Ľṹ��Ԫ����ͼ��ʾ����³ʿ���� n(K+)��n(Fe3+)��n(Fe2+)��n(CNһ)= _____ ___��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�Ͼ�һģ���������Ļ�������������������������Ҫ����;��
��2011?�Ͼ�һģ���������Ļ�������������������������Ҫ����;��2- 2 |
2+ 2 |
2- 2 |
2+ 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���������Ļ�������������������������Ҫ����;��
��1���������Ļ�������������������������Ҫ����;���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������������и�����ѧ��11�·��¿���ѧ�Ծ� ���ͣ������
��16�֣���1���������Ļ�������������������������Ҫ����;��
�١��ۺ������������PFS��[Fe ��OH��n��SO4����3-n�� / 2 ]m��m�Ǿۺ϶ȣ�����������ˮ�����γ���״��������ؽ������ӡ���������Ԫ�صļ�̬Ϊ__________��SO42-�Ŀռ乹��Ϊ ��
�ڡ�����������K3[Fe��CN��6]�����ڼ���Fe2+�������������ɫ������CN����̼ԭ�ӵ��ӻ���ʽΪ__________,д����CNһ��Ϊ�ȵ������һ�����ӵĻ�ѧʽ_______,�����ʽΪ________��
�����Ȼ����ڳ�����Ϊ���壬�۵�304�棬�е�316�棬300�����Ͽ�������������ˮ��Ҳ���������ѣ���ͪ���л��ܼ����ݴ��ƶ����Ȼ�������Ϊ__________���塣
����³ʿ����һ������������Ⱦ�ϣ����Ľṹ��Ԫ����ͼ��ʾ����³ʿ����n��CNһ����n��K+����n��Fe3+����n��Fe2+�� = ____________��
��2��ˮ�Ͱ�������֮�����磺H2O+H+=H3O+ ��NH3+H+=NH4+��
��NH4+�ļ���__________H3O+�ļ��ǣ�����ڡ���С�ڡ�����
�ڱ���̬���ľ����������ڷ������ܶѻ���ԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡʦ���и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣��������Ļ������������������������� Ҫ����;��
Ҫ����;��
(1)�ۺ�������(���PFS)�Ļ�ѧʽΪ [ Fe (OH)n(S04) (3-n) / 2 ]m ���ִ�DZˮ���������г�����PFS��ˮ�����γ���״�� ���������ؽ������ӡ���PFS������ԭ��δ�ɶԵ�����Ϊ__ ����
���������ؽ������ӡ���PFS������ԭ��δ�ɶԵ�����Ϊ__ ����
(2)������������K4[Fe(CN)6]��������Ӱ�����û������д��ڵĻ�ѧ��������_____  ____ ��
____ ��
��3��CNһ��̼ԭ�ӵ��ӻ���������� _______ ��д��һ����CNһ��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ_____ ___��
(4)���Ȼ���������Ϊ���壬�۵�304�棬�е�3160C ��3000C���Ͽ�������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж����Ȼ�������Ϊ______ ���塣
(5)��³ʿ����һ������������Ⱦ�ϣ����Ľṹ��Ԫ����ͼ��ʾ����³ʿ���� n(K+)��n(Fe3+)��n(Fe2+)��n(CNһ)= _____ ___��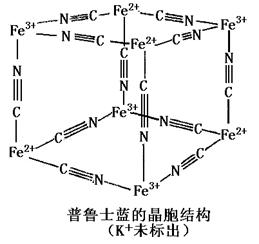
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com