
(10·Ö£©
2006ÄźŹĄ½ēļ®Ąė×Óµē³Ų×ܲśĮ泬¹ż25ŅŚÖ»£¬ļ®µē³ŲĻūŗÄĮ澎“󣬶Ō²»æÉŌŁÉśµÄ½šŹō׏Ō“µÄĻūŗÄŹĒĻąµ±“óµÄ”£Ņņ“Ėļ®Ąė×Óµē³Ų»ŲŹÕ¾ßÓŠÖŲŅŖŅāŅ壬ĘäÖŠŠčŅŖÖŲµć»ŲŹÕµÄŹĒÕż¼«²ÄĮĻ£¬ĘäÖ÷ŅŖ³É·ÖĪŖīÜĖįļ®£ØLiCoO2£©”¢µ¼µēŅŅČ²ŗŚ(Ņ»ÖÖĢæŗŚ£©”¢ĀĮ²ŅŌ¼°ÓŠ»śÕ³½Ó¼Į”£Ä³»ŲŹÕ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
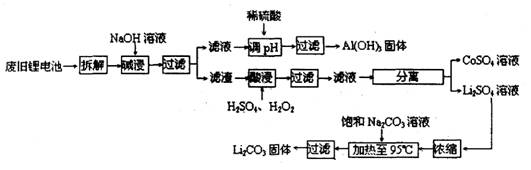
£Ø1£©ÉĻŹö¹¤ŅÕ»ŲŹÕµ½µÄ²śĪļÓŠ
£Ø2£©LiŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ £¬·Ļ¾Éµē³ŲæÉÄÜÓÉÓŚ·Åµē²»ĶźČ«¶ų²ŠĮōÓŠŌ×ÓĢ¬µÄļ®£¬ĪŖĮĖ°²Č«¶Ō²š½ā»·¾³µÄŅŖĒóŹĒ___________________________________________
£Ø3£©¼ī½žŹ±Ö÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©×īŗóŅ»²½¹żĀĖÓ¦³ĆČČ¹żĀĖ£¬ŌŅņŹĒ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø07Äź½ĖÕ¾ķ£©(10·Ö)øÖĢś¹¤ŅµŹĒ¹ś¼Ņ¹¤ŅµµÄ»ł“””£2006ÄźĪŅ¹ś“ÖøÖ²śĮæĶ»ĘĘ4ŅŚ¶Ö£¬¾ÓŹĄ½ēŹ×Ī»”£Ä³ÖŠŃ§Éē»įŹµ¼ł»ī¶ÆŠ”×éĄūÓĆ¼ŁĘŚ¶Ōµ±µŲøÖĢś³§½ųŠŠĮĖµ÷ŃŠ£¬¶Ō“ÓæóŹÆæŖŹ¼µ½øÖĢś²ś³öµÄ¹¤ŅÕĮ÷³ĢÓŠĮĖČ«ĆęµÄøŠŠŌČĻŹ¶”£ĒėÄś¶ŌÉē»įŹµ¼ł»ī¶ÆŠ”×éøŠŠĖȤµÄĪŹĢā½ųŠŠ¼ĘĖć£ŗ
(1)½«6£®62 gĢśæóŹÆѳʷĶ¶ČėŹŹĮæµÄŃĪĖįÖŠ(³ä·Ö·“Ó¦)£¬¹żĀĖ£¬Č»ŗóŌŚĀĖŅŗÖŠ¼Ó¹żĮæµÄNaOH ČÜŅŗ£¬³ä·Ö·“Ó¦ŗ󣬹żĀĖ”¢Ļ“µÓ”¢×ĘÉÕµĆ4.80g Fe2O3”£ĻÖŅŌøĆĢśæóŹÆĪŖŌĮĻĮ¶Ģś£¬ČōÉś²ś¹ż³ĢÖŠĢśŌŖĖŲĖšŹ§4£„£¬¼ĘĖćĆæÉś²ś1.00tÉśĢś(ŗ¬Ģś96£„)£¬ÖĮÉŁŠčŅŖÕāÖÖĢśæóŹÆ¶ąÉŁ¶Ö? (±£ĮōĮ½Ī»Š”Źż)
(2)Č”Ä³øÖŃł·ŪÄ©28.12g(¼ŁÉčÖ»ŗ¬FeŗĶC)£¬ŌŚŃõĘųĮ÷ÖŠ³ä·Ö·“Ó¦£¬µĆµ½CO2ĘųĢå224mL(±ź×¼×“æö)”£
¢Ł ¼ĘĖć“ĖøÖŃł·ŪŹõÖŠĢśŗĶĢ¼µÄĪļÖŹµÄĮæÖ®±Č”£
¢ŚŌŁČ”Čż·Ż²»Ķ¬ÖŹĮæµÄøÖŃł·ŪÄ© ·Ö±š¼Óµ½100mLĻąĶØÅØ¶ČµÄH2SO4ČÜŅŗÖŠ£¬³ä·Ö·“ Ó¦ŗó£¬ ²āµĆµÄŹµŃ鏿¾ŻČēĻĀ±ķĖłŹ¾£ŗ
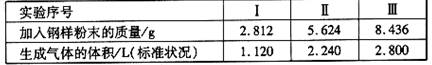 |
¼ĘĖćĮņĖįČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č”£
¢ŪČōŌŚŹµŃé¢ņÖŠ¼ĢŠų¼ÓČėmgøÖŃł·ŪÄ©£¬¼ĘĖć·“Ó¦½įŹųŗóŹ£ÓąµÄ¹ĢĢåÖŹĮæĪŖ¶ąÉŁ?(ÓĆŗ¬mµÄ“śŹżŹ½±ķŹ¾)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¾ŻÖŠ¹ś·ÄÖÆĮ÷ĶØŠ»įŹż¾ŻĶ³¼Ę£¬2006Äź8ŌĀ·ŻČ«¹śÉś²śĪ¬ĀŚĻĖĪ¬3424¶Ö£¬ÓėČ„ÄźĶ¬±ČŌö³¤10.59%”£Ī¬ĀŚµÄ³É·ÖŹĒ¾ŪŅŅĻ©“¼Ėõ¼×Č©£¬ĖüæÉŅŌÓÉŹÆÓĶµÄ²śĘ·ŅŅĻ©ĪŖĘšŹ¼ŌĮĻ½ųŠŠŗĻ³É£¬ĘäÖ÷ŅŖ²½ÖčŹĒÓÉŅŅĻ©”¢ŃõĘųŗĶ“×ĖįŗĻ³Éõ„ĖįŅŅĻ©õ„£¬»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
CH2==CH2 + O2 + CH3COOH CH3COOCH==CH2+ H2O
Č»ŗóŌŁ¾¹ż¼Ó¾Ū”¢Ė®½ā”¢ĖõŗĻÖʵĆĪ¬ĀŚ”£
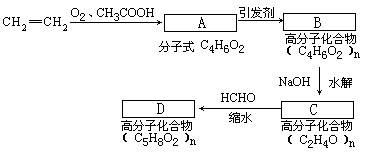
(1) A”śBµÄ·“Ó¦ĄąŠĶĪŖ________________”£
(2)ĒėČ·¶ØA”¢B”¢DµÄ½į¹¹¼ņŹ½£ŗ
A£ŗ________________B£ŗ________________ D£ŗ_______________”£
(3) Š“³öCµÄµ„ĢåµÄµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(10·Ö£©
2006ÄźŹĄ½ēļ®Ąė×Óµē³Ų×ܲśĮ泬¹ż25ŅŚÖ»£¬ļ®µē³ŲĻūŗÄĮ澎“󣬶Ō²»æÉŌŁÉśµÄ½šŹō׏Ō“µÄĻūŗÄŹĒĻąµ±“óµÄ”£Ņņ“Ėļ®Ąė×Óµē³Ų»ŲŹÕ¾ßÓŠÖŲŅŖŅāŅ壬ĘäÖŠŠčŅŖÖŲµć»ŲŹÕµÄŹĒÕż¼«²ÄĮĻ£¬ĘäÖ÷ŅŖ³É·ÖĪŖīÜĖįļ®£ØLiCoO2£©”¢µ¼µēŅŅČ²ŗŚ(Ņ»ÖÖĢæŗŚ£©”¢ĀĮ²ŅŌ¼°ÓŠ»śÕ³½Ó¼Į”£Ä³»ŲŹÕ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
£Ø1£©ÉĻŹö¹¤ŅÕ»ŲŹÕµ½µÄ²śĪļÓŠ
£Ø2£©LiŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ £¬·Ļ¾Éµē³ŲæÉÄÜÓÉÓŚ·Åµē²»ĶźČ«¶ų²ŠĮōÓŠŌ×ÓĢ¬µÄļ®£¬ĪŖĮĖ°²Č«¶Ō²š½ā»·¾³µÄŅŖĒóŹĒ___________________________________________
£Ø3£©¼ī½žŹ±Ö÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©×īŗóŅ»²½¹żĀĖÓ¦³ĆČČ¹żĀĖ£¬ŌŅņŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”ŹµŃé֊ѧøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(10·Ö£©
2006ÄźŹĄ½ēļ®Ąė×Óµē³Ų×ܲśĮ泬¹ż25ŅŚÖ»£¬ļ®µē³ŲĻūŗÄĮ澎“󣬶Ō²»æÉŌŁÉśµÄ½šŹō׏Ō“µÄĻūŗÄŹĒĻąµ±“óµÄ”£Ņņ“Ėļ®Ąė×Óµē³Ų»ŲŹÕ¾ßÓŠÖŲŅŖŅāŅ壬ĘäÖŠŠčŅŖÖŲµć»ŲŹÕµÄŹĒÕż¼«²ÄĮĻ£¬ĘäÖ÷ŅŖ³É·ÖĪŖīÜĖįļ®£ØLiCoO2£©”¢µ¼µēŅŅČ²ŗŚ(Ņ»ÖÖĢæŗŚ£©”¢ĀĮ²ŅŌ¼°ÓŠ»śÕ³½Ó¼Į”£Ä³»ŲŹÕ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ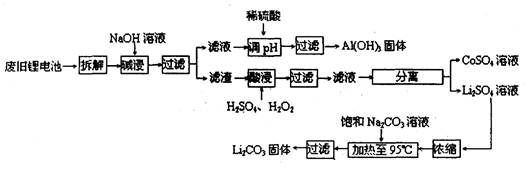
£Ø1£©ÉĻŹö¹¤ŅÕ»ŲŹÕµ½µÄ²śĪļÓŠ
£Ø2£©LiŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ £¬·Ļ¾Éµē³ŲæÉÄÜÓÉÓŚ·Åµē²»ĶźČ«¶ų²ŠĮōÓŠŌ×ÓĢ¬µÄļ®£¬ĪŖĮĖ°²Č«¶Ō²š½ā»·¾³µÄŅŖĒóŹĒ___________________________________________
£Ø3£©¼ī½žŹ±Ö÷ŅŖ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©×īŗóŅ»²½¹żĀĖÓ¦³ĆČČ¹żĀĖ£¬ŌŅņŹĒ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com