
ijͬѧ��һֻ�ձ���װ��a g�����ۣ�����200 mL��6 mol/L����ǡ����ȫ�ܽ�����(���軹ԭ����ֻ��NO)������ɶԲ�������Ԫ�ؼ�̬��̽����
(1)����������裺
����1������ֻ�У�2������
����2��________��
����3��________��
(2)���ʵ�飺�ֱ�ȡ��Ӧ����Һװ����֧�Թܼס��ң��ڼ��Թ��еμ����Ը��������Һ�������Թ��еμ�KSCN��Һ���۲�����
�Ʋ�ʵ����������ۣ���������Ϊ��________��
�����1��ȷ����������Ϊ��________��
�����2��ȷ����������Ϊ��________��
�����3��ȷ��
(3)aֵ��ΧΪ________��
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �۲�Ҫ���ʵ�鲽�� | �����ۻ�ע������ | |
| ����һ | �۲��������ϵ�ϸС��� | �� ��ɫ��ĩ ��ɫ��ĩ ״����̼���ƣ�����ɫϸС���� ��ɫϸС���� ����̼������ |
| ������ | �ֱ���뼸��ˮ | ��ɿ�״�����Թܵײ��¶Ƚϸߵ��� ̼���� ̼���� ��δ��鲢���Թܵײ��¶Ƚϵ͵��� ̼������ ̼������ �� |
| ������ | �ٷֱ����10mLˮ������ 1��2�η�̪��Һ |
�ò����м���ˮ��Ӧ �������ò��������� �������ò��������� �������̪��Ӧ���ع۲� ������Һ��ɫ����dz ������Һ��ɫ����dz �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
��������ƽ���˸���һֻ�ձ�, ������ƽ��. �����ձ���ֱ�ע��������� ���������������ҹ�����ϡ����, Ȼ����һֻ�ձ������һ��������þ, ���� һ�ձ�������������ͭ���Ͻ�, ���ձ��ķ�Ӧ��ɺ�, ��ƽ�Ա���ƽ��. �� ͭ���Ͻ���ͭ������������Ϊ
[����]
A. 1:3 ���� B. 1:2 ���� C. 3:4��������D. 2:3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и�����һ�ε��п��������ۺϻ�ѧ�Ծ����������� ���ͣ�ʵ����
��16�֣���һ��ijͬѧ��һֻ�ձ���װ��һ�����Ĵ����ۣ�����200mL 6mol/L�����ᣬ����ǡ���ܽ⣬��̽����������Ԫ�ؼ�̬��
(1)������裺
����1������ֻ��+2������
����2:___________________________________��
����3:___________________________________��
(2)���ʵ�飺ȡ��Ӧ������Һ�ֱ�װ��ס�����֧�Թܣ��ڼ��еμ�����KMnO4��Һ�������еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ____________________�������1��ȷ��
��������Ϊ____________________�������2��ȷ��
��������Ϊ____________________�������3��ȷ��
���������Ȼ����dz�����ˮ����������ҵ���Ʊ���ˮFeCl3������Ϊ��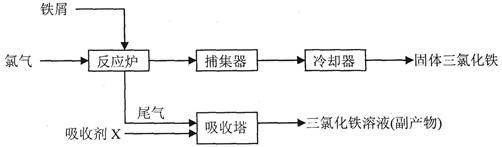
(3)���ռ�XΪFeCl2��Һ������β��Cl2��Ӧ�����ӷ���ʽ__________________��
(4)��ȡ������Ʒm������25mLϡ���ᣬ������ˮ���50mL��Һ�������Թ�����KI��Һ��ַ�Ӧ�� ���õ�����ָʾ������
���õ�����ָʾ������ ��Һ���еζ�
��Һ���еζ�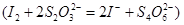 ������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
(5)��FeCl3��Һ(32%-35%)��ʴͭ���·ʱ���÷�Һ��FeCl3��FeCl2��CuCl2�����û�ѧ�������շ�Һ�е�ͭ����������Ҫ�㣺___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����и�����һ�ε��п��������ۺϻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
��16�֣���һ��ijͬѧ��һֻ�ձ���װ��һ�����Ĵ����ۣ�����200mL 6mol/L�����ᣬ����ǡ���ܽ⣬��̽����������Ԫ�ؼ�̬��
(1)������裺
����1������ֻ��+2������
����2:___________________________________��
����3:___________________________________��
(2)���ʵ�飺ȡ��Ӧ������Һ�ֱ�װ��ס�����֧�Թܣ��ڼ��еμ�����KMnO4��Һ�������еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ____________________�������1��ȷ��
��������Ϊ____________________�������2��ȷ��
��������Ϊ____________________�������3��ȷ��
���������Ȼ����dz�����ˮ����������ҵ���Ʊ���ˮFeCl3������Ϊ��

(3)���ռ�XΪFeCl2��Һ������β��Cl2��Ӧ�����ӷ���ʽ__________________��
(4)��ȡ������Ʒm������25mLϡ���ᣬ������ˮ���50mL��Һ�������Թ�����KI��Һ��ַ�Ӧ�� ���õ�����ָʾ������
���õ�����ָʾ������ ��Һ���еζ�
��Һ���еζ� ������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
(5)��FeCl3��Һ(32%-35%)��ʴͭ���·ʱ���÷�Һ��FeCl3��FeCl2��CuCl2�����û�ѧ�������շ�Һ�е�ͭ����������Ҫ�㣺___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com