
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��| 1 |
| 2 |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ӧ����Һ��Cu2+��Fe2+�����ʵ���֮��Ϊ1��2 |
| B�������ԣ�Ag+��Cu2+��Fe3+��Zn2+ |
| C����Fe3+����Һ�ɸ�ʴͭ�� |
| D��1mol Fe�ɻ�ԭ2mol Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe2+��NO3-��Cl-��H+ |
| B��Ca2+��Al3+��Cl-��HCO3- |
| C��Mg2+��Al3+��Cl-��SO42- |
| D��Na+��Ba2+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ���ձ��е����ʾ�������a��b��c��d��e��Ϊʯī�缫��ͨ��һ��ʱ���a�缫���к�ɫ��������������˵����ȷ���ǣ�������
��ͼ���ձ��е����ʾ�������a��b��c��d��e��Ϊʯī�缫��ͨ��һ��ʱ���a�缫���к�ɫ��������������˵����ȷ���ǣ�������| A��e�缫�Ͽ�������ʹʪ��ĵ���KI��ֽ���������� |
| B��c�缫��Χ��Һ��� |
| C��A�ձ���Cu2+Ũ�Ƚ��� |
| D��B�ձ��м�һ������KCl����ɻָ�ԭ״ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol O2��Ϊ�������õ��ĵ�����һ��Ϊ4NA |
| B���ڱ�״���£�11.2Lˮ�к��з�����Ϊ0.5NA |
| C��0.4mol/L Na2SO4��Һ�У�����Na+��SO42-����Ϊ1.2NA |
| D����״���£�40g SO3��ռ�����һ��С��11.2L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
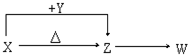 X��Y��Z��W���ֳ������������X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ������������ת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ����
X��Y��Z��W���ֳ������������X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ������������ת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com