
��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺
��1��M�缫�IJ������������������� ���缫���������������� ��N�ĵ缫��ӦʽΪ��������������������ͨ���Ҵ��IJ��缫�ĵ缫��ӦʽΪ������������������������ ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ��������������������L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ�������������������� ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ������������������������������ ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺
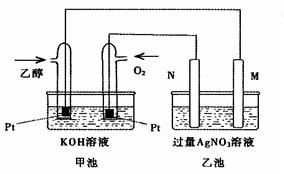
��1��M�缫�IJ����� ���缫������ ��N�ĵ缫��ӦʽΪ �������Ҵ��IJ��缫�ĵ缫��Ӧʽ ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ���㽭ʡ����ʦ����ѧ������ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��9�֣���״�ȼ�ϵ����ȣ��Ҵ�ȼ�ϵ�ؾ��ж��Ե͡����������ܶȸߵ��ŵ㣬��˱��㷺��Ϊ�Ǹ���ǰ;��ȼ�ϵ�ء���ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫��Ϊʯī�缫���ҳ���ʢ��100 mL3.00 mol/L��CuSO4��Һ����ش��������⣺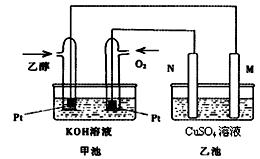
(1) �ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�30kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
(2)N�ĵ缫��ӦʽΪ ��
(3)�ڴ˹����У��ҳ���ijһ�缫��������ͭ6.4gʱ���׳����������������� ��
����״���£�
(4) �ڴ˹����У����ҳ������缫����������ǡ�����ʱ(�����״�� ��)����������ͨ���Ҵ� g��
��)����������ͨ���Ҵ� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ��ʮ���и�����ѧ��11���¿���ѧ�� ���ͣ������
��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺
��1��M�缫�IJ����� ���缫������ ��N�ĵ缫��ӦʽΪ �������Ҵ��IJ��缫�ĵ缫��Ӧʽ ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4.32gʱ���׳�����������������Ϊ L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29.71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
��ͼ��һ���Ҵ�ȼ�ϵ�س��¹���ԭ��ʾ��ͼ���ҳ��е������缫һ��
��ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�������˵����ȷ����
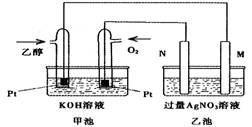 A.M�缫�IJ�����ʯī
A.M�缫�IJ�����ʯī
B.���ҳ���ijһ�缫��������4.32gʱ����������������Ϊ448ml
C.�ڴ˹����У��׳���OH-��ͨ�Ҵ���һ���ƶ�
D.�ڴ˹����У��ҳ���Һ�е��Ӵ�M�缫��N�缫�ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��9�֣���״�ȼ�ϵ����ȣ��Ҵ�ȼ�ϵ�ؾ��ж��Ե͡����������ܶȸߵ��ŵ㣬��˱��㷺��Ϊ�Ǹ���ǰ;��ȼ�ϵ�ء���ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫��Ϊʯī�缫���ҳ���ʢ��100 mL3.00 mol/L��CuSO4��Һ����ش��������⣺
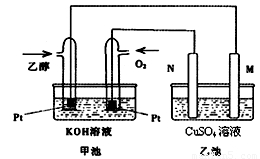
(1) �ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�30kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
(2)N�ĵ缫��ӦʽΪ ��
(3)�ڴ˹����У��ҳ���ijһ�缫��������ͭ6.4gʱ���׳����������������� ��
����״���£�
(4) �ڴ˹����У����ҳ������缫����������ǡ�����ʱ(�����״����)����������ͨ���Ҵ� g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com