

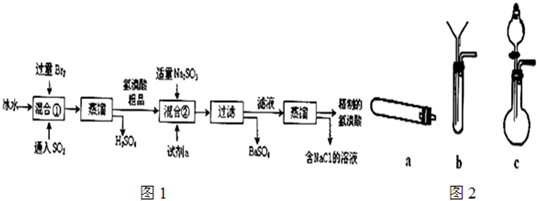
 2NaHSO4+MnSO4+Br2��+2H2O ��ȡBr2���ѡ������װ���е�______ ����д��ĸ���̶��ͼ���װ�þ���ʡ�ԣ�����������ͼ2װ�������Եķ���______��
2NaHSO4+MnSO4+Br2��+2H2O ��ȡBr2���ѡ������װ���е�______ ����д��ĸ���̶��ͼ���װ�þ���ʡ�ԣ�����������ͼ2װ�������Եķ���______��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
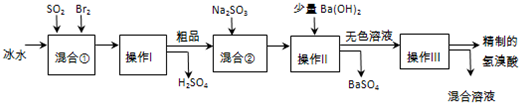
��16�֣���������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ���������̣�
�����������̻ش��������⣺
��1����Ϣ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ϣ���ʹ�ñ�ˮ��Ŀ���� ��
��3��������Ͳ���������Ʒֱ��� �� ��
������һ�������ڷ���____________������ѡ���ţ�
a�������Һ�� b��������� c���������ܵ�Һ�� d�����ܵ�Һ��
��4����Ϣ��м���Na2SO3��Ŀ���� ��
��5��������������ӦΪ��ɫҺ�壬��ʵ�ʹ�ҵ�������Ƶõ������ᣨ��ҵ�����ᣩ���е����Ļ�ɫ�����Ǽ�����ͬѧ����˼�ʵ�����̽����
��ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3����������֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
��ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ_______��������֤���ü������õ��Լ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
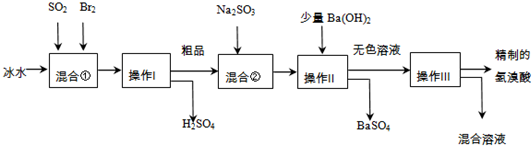
(15��)��������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ���������̣�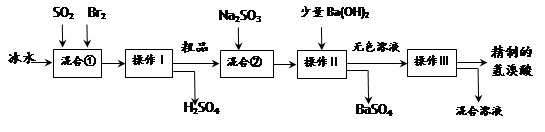
�����������̻ش��������⣺
��1����Ϣ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ϣ���ʹ�ñ�ˮ��Ŀ���� ��
��3��������Ͳ���������Ʒֱ��� �� ��
������һ�������ڷ���____________������ѡ���ţ�
a�������Һ�� b��������� c���������ܵ�Һ�� d�����ܵ�Һ��
��4����Ϣ��м���Na2SO3��Ŀ���� ��
��5��������������ӦΪ��ɫҺ�壬��ʵ�ʹ�ҵ�������Ƶõ������ᣨ��ҵ�����ᣩ���е����Ļ�ɫ�����Ǽ�����ͬѧ����˼�ʵ�����̽����
��ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ��Fe3������ ����֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
����֤���ü������õ��Լ�Ϊ ������������ɹ۲쵽������Ϊ ��
��ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ________��������֤���ü������õ��Լ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com