
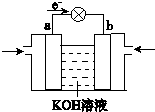
| 1 |
| 2 |
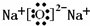
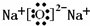
| 1 |
| 2 |
| 1 |
| 2 |


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������Q�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Q��W��ɵĻ�������һ���������壬W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�Y��Z���γ�ԭ�Ӹ�����Ϊ1��1��1��2���������ӻ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
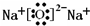
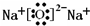
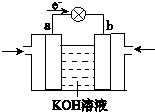
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮
Q��W��X��Y��Z��5�ֶ�����Ԫ�أ�ԭ������������W��Q��1��4�γɵĻ�����A��һ����Ҫ��Դ��W��Y��X��Y��ɵĻ������dz����Ĵ�����Ⱦ�Y��Z��1��1�γɵ����ӻ�����B����Ħ������Ϊ78g?mol-1����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���⻯���ȶ��ԣ�Z��W | B��ԭ�Ӱ뾶�Ĵ�С˳��rX��rY��rQ��rW | C��Ԫ��Q��Z���γ�QZ2�͵Ĺ��ۻ����� | D��X��Y������������ˮ����֮�䲻�ܷ�����Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com