
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij�����м���ϡ���ᣬ������ʹ����ʯ��ˮ����ǵ����� | ˵������һ����̼���� |
| B | ��ij��ɫ��Һ�еμ�NaOH��Һ���Ȳ�����ɫ������������ֻ�ȫ����ʧ | ����ɫ��Һ��һ����Al3������Mg2�� |
| C | ����Һ�м�������Cu(OH)2��û��ש��ɫ�������� | ˵����Һ�в����������� |
| D | ij����Һ���ȵ�����ˮ����������������軯����Һ����Ѫ��ɫ | �ô���Һ��һ������Fe2�� |
 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
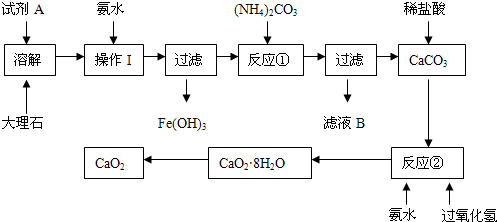
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
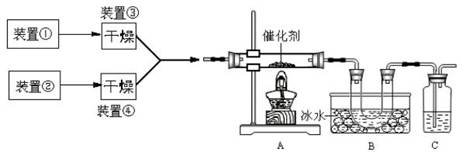

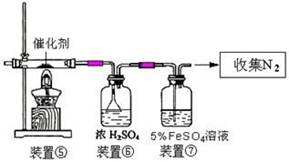
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+ | B��SO42�� | C��Ba2+ | D��NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
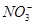 ��
�� ��Cl����I����
��Cl����I���� ��ȡ����Һ����ʵ�飺
��ȡ����Һ����ʵ�飺| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
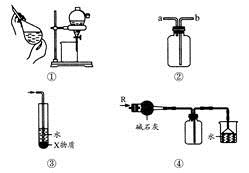
| A��װ�âٿ����ڷ���C2H5OH��H2O�Ļ���� |
| B��װ�âڿ������ռ�H2��NH3��CO2��Cl2��HCl��NO2������ |
| C��װ�â���X��ΪCCl4������������NH3��HCl������ֹ���� |
| D��װ�âܿ����ڸ���ռ�NH3�������ն����NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
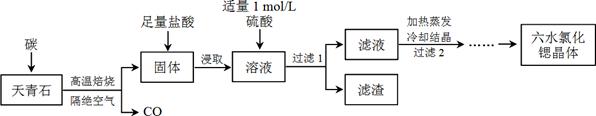
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �� �� | ˮ | �Ҵ� | ���� | �� | �������� |
| �е㣯�� | 100 | 78.4 | 122 | 80.10 | 154 |
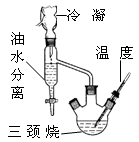
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com