
����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450ml��0.5mol/L������Һ500ml��������������Һ����������ش��������⣺
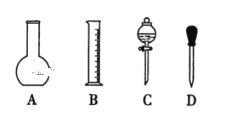
��1����ͼ��ʾ�������У�������Һ�϶�����Ҫ����________������ţ�������������Һ�����õ��IJ���������________�����������ƣ���
��2������0.1mol/L��NaOH��Һ�IJ����������£���ȷ��˳����_______��
�ٰѳ����õ�NaOH�������С�ձ��У�������������ˮ�ܽ⣻
������������ˮϴ���ձ��Ͳ�����2~ 3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ�У�������ҡ�ȣ�
�ۼ���������ƿ�м�����ˮ��Һ���̶���1~ 2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ�İ�Һ����̶������У�
�ܰѢ�������Һ��ȴ�����£���С��ת��һ���ݻ�������ƿ�У�
�ݽ�����ƿƿ�����������ҡ�ȡ�
��3�����ݼ��㣬��������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��________0.1mol/L��������������С������������������ͬ��������δ����Һ��ȴ�Ͷ��ݣ���������ҺŨ��________0.1mol/L�����ƺú��ֳ���ʱ���õ�������ƽ������������________0.1mol/L��
���𰸡�A C 500ml����ƿ�����������ձ� �٢ܢڢۢ� 2.0 ���� ���� ����
��������
��1�����Ʋ����м��㡢��������ȡ�����ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ��������ݲ����ж���ѡ������
��2������ʵ������ʵ�鲽���г������ܽ⡢��Һ��ϴ�ӡ������Լ�ҡ�ȵȲ�����
��3������n=cV��m=nM�����������Ƶ�������
����c=![]() ���
���
��1�����Ʋ����м��㡢��������ȡ�����ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����������Ϊ���壬һ����������ƽ��������ҩ��ȡ��ҩƷ��������ΪҺ�壬һ������Ͳ��ȡ��Ȼ�����ձ����ܽ⣬��ȴ��ת�Ƶ���Ӧ��������ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ����Բ���Ҫ��������AC������Ҫ��������500mL����ƿ�����������ձ���
��2������ʵ������ʵ�鲽���г������ܽ⡢��Һ��ϴ�ӡ������Լ�ҡ�ȵȲ���������ȷ��˳���ǣ��٢ܢڢۢݣ�
��3��������450mL������ƿ����ѡ��500mL������ƿ����Ҫ�������Ƶ����ʵ���n=cV=0.50mol/L��0.5L=0.25mol������Ϊm=nM=0.25mol��40g/mol=2.0g��
��ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ������������ҺŨ����Ӱ�죬����0.1mol/L��
����δ����Һ��ȴ�Ͷ��ݣ���ȴ����Һ�������С����������ҺŨ�ȴ���0.1mol/L��
���ƺú��ֳ���ʱ���õ�������ƽ�����������ˣ����������Ĺ�������ƫ��������Һ��Ũ��ƫ�������0.1mol/L��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҺX�п��ܺ������������е������֣�Cl-��SO42-��SO32-��HCO3-��Na+��Mg2+��Fe3+���������ӵ����ʵ���Ũ�Ⱦ���ͬ��Ϊ��ȷ������Һ����ɣ�ijͬѧȡ100mL������ҺX������������ʵ�飺(1)����ҺX�м���������Ba(OH)2��Һ���õ���ɫ������(2)��(1)�ķ�Ӧ���Һ���ˣ������������������У����������ܽ��Ҳ������塣
����˵����ȷ����![]()
![]()
A. (2)������������CO2��SO2
B. ��ҺX��һ������SO42-��HCO3-��Mg2+
C. ��ҺX��һ��������Fe3+�����ܴ���Cl-
D. (1)�в����İ�ɫ����һ������BaSO4�����ܺ���BaSO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����Է�������Ϊ86�������ķ���ʽ�� _________��
��2����ϵͳ������������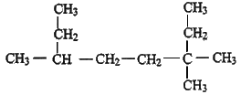 _______________________��
_______________________��
��3����ϵͳ������������ 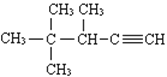 ______________________________��
______________________________��
��4��˳��2����ϩ�Ľṹ��ʽΪ_________________________��
��5��̼ԭ����1��10��������һ�ȴ���ֻ��һ�ֵ�������4�֣����ǵĽṹ��ʽΪCH4��CH3CH3��_____________________��_____________________��
��6��ʵ������ȡ��Ȳ�Ļ�ѧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ҺX����K����Mg2����Fe3����Al3����Fe2����Cl����CO32����OH����SiO32����NO3����SO42���еļ��֣���֪����Һ�и��������ʵ���Ũ�Ⱦ�Ϊ0.20mol��L��1��������ˮ�ĵ��뼰���ӵ�ˮ�⣩��Ϊȷ������Һ�к��е����ӣ��ֽ��������µIJ�����
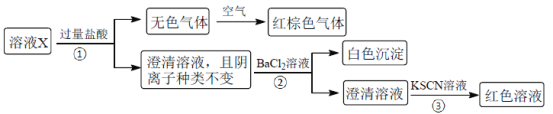
����˵����ȷ����
A.��ɫ���������NO��CO2�Ļ����
B.�ɲ���ۿ�֪��ԭ��Һ�϶�����Fe3��
C.��ҺX���������������4��
D.��ȡ100mLԭ��ҺX������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ������ϵõ��Ĺ�������Ϊ2.4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaC1O2��һ����Ҫ��ɱ����������Ҳ������Ư��֯��ȣ���һ�������������£�

��1��д������Ӧ������������ClO2�Ļ�ѧ����ʽ��______________��
��2�� ����⡱��������Ӧ����Ҫ������_______________________��
��3�������������ʳ��ˮ�ɴ���ˮ���ƶ��ɣ�����ʱ��Ϊ��ȥMg2+��Ca2+��Ҫ������Լ��քeΪ______________��Һ��______________��Һ���ѧʽ����
��4����β��������������������������ų�������ClO2��д����β�������������ӷ���ʽ��_______________________�������շ�Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ________��
��5������Ч�Ⱥ��������������������������������������䶨���ǣ�ÿ�˺��������������������൱�ڶ��ٿ�Cl2������������NaClO2����Ч�Ⱥ���Ϊ____g��������������λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ԭ��Ӧ�У�ˮ��Ϊ����������
A. CO��H2O![]() CO2��H2 B. 3NO2��H2O��2HNO3��NO
CO2��H2 B. 3NO2��H2O��2HNO3��NO
C. 2Na2O2��2H2O��4NaOH��O2�� D. 2F2��2H2O��4HF��O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������Ҫ������NaCl��Һ�����ֱ�ֻ�л���Na2SO4��NH4HCO3��NaCl��ijѧ���������ͼ��ʾ������ȡ������NaCl��Һ��(��֪��NH4HCO3=NH3����CO2����H2O)

��1��������Ϊʲô�������ᱵ��Һ����������_______________________________��
��2�����в�����������ж�SO42-�ѳ�����������___________________________��
��3����������Ŀ����____________�������漰�������ӷ���ʽ��_______________��
��4������Һ�м�����������ӷ���ʽ��__________________________________��
��5��NH4HCO3��ˮ��Һ�еĵ��뷽��ʽ_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ�������ж���ȷ����
A. ��״���£�2.24L���к���̼̼˫����Ϊ0.3NA
B. 6.4gS2��S4��S8�Ļ������������ԭ����Ϊ0.2NA
C. 1L0.1mol��L-1��������к��⻯�������Ϊ0.1NA
D. 60g�����������Ҵ�����������Ӧʱ���ѵ�C-O����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Դ�ı��⣬����ʮ�־�Ŀ���DZ������ҵ�ϴӺ�ˮ��ȡ����þ���������£�
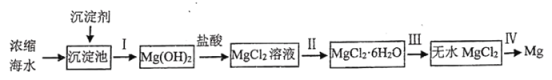
�ش��������⣺
��1����������________________���ѧʽ������ѡ��ͬ����������ʵ�������_______________��
��2������ѡ���У��漰�IJ����������������___________��
A ʵ���Ҵ���ˮ����ȡ�嵥��
B ��ȥ![]() �����е�����NaCl
���������NaCl
C ��![]() �ֽ�����л��
�ֽ�����л��![]()
D �ù�ҵ�ƾ�����ˮ�Ҵ�
��3���ڽ��в����ʱ�����û�м������ᣬ���ܲ�����������______________���ѧʽ����
��4��һ��ģ�ҵ��![]() ����ˮ
����ˮ![]() ��ʵ����������£�
��ʵ����������£�
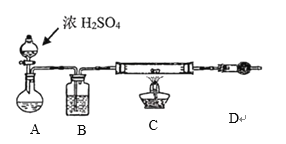
װ��A��ʢ��Һ����______________��װ��D��������____________________________��
��5�������������Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com