
��ͼ��ʾ���ϳɰ�������ʾʵ��(�г���������ʡ��)����Y�ιܵ�һ����Zn����ϡH2SO4��Ӧ��ȡH2����һ����NaNO2�����NH4Cl������Һ��Ӧ��ȡN2��N2��H2��Ϻ�ͨ����ԭ�������ϳ�NH3���ٽ�����������ͨ���̪��Һ�У�����̪��Һ��죬��˵�������˰�����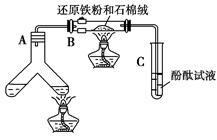
ij����С��ͨ���������ϺͶ��ʵ�飬�õ���������Ϣ��
��Ϣһ��NaNO2����ͱ���NH4Cl��Һ��ϼ��ȵĹ����з������·�Ӧ��
��NaNO2��NH4Cl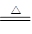 NH4NO2��NaCl
NH4NO2��NaCl
��NH4NO2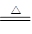 NH3����HNO2
NH3����HNO2
��2HNO2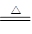 N2O3����H2O
N2O3����H2O
��2NH3��N2O3 2N2��3H2O
2N2��3H2O
��Ϣ�����������ϣ���ͬ����ȵ�N2��H2�����������ͬʵ�������ºϳɰ���ʹ��̪��Һ�������Ҫ��ʱ�����£�
| N2��H2������� | 5��1 | 3��1 | 1��1 | 1��3 | 1��5 |
| ��̪���ɫ����ʱ��/min | 8��9 | 7��8 | 6��7 | 3��4 | 9��10 |
 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л�ѧʵ�������ܹ��ﵽĿ�ĵ���
| A��Ϊ����KCl��AlCl3��MgCl2��Һ���ֱ���������Һ�еμ�NaOH��Һ������ |
| B������ȥ��������Һ�е�NaCl���ֲ��ı������ʣ��ɼ�������BaCl2��Һ����� |
| C������ˮ��pH�����ò�����պȡ��ˮ����pH��ֽ�ϣ������ɫ��ͱ���ɫ���Ƚ� |
| D��Ϊ��֤����¯���к����������ɽ���¯��ͨ�����ȵ�����ͭ��ĩ������ɫ��ĩ�Ƿ���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������(NH2COONH4)��һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ�ҵԭ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�2 NH3(g)+CO2(g)  NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0 
��1��ʵ�����Ʊ�NH3�Ļ�ѧ����ʽ�ǣ� ��
��2���Ʊ���������淋�װ������ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С���������϶�ʱ��ֹͣ�Ʊ���
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ���� ��Һ��ʯ������ƿ�������� ��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���� (��д��������)��Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ����� (��дѡ�����)��
a. ��ѹ���Ⱥ�� b. ��ѹ���Ⱥ�� c. ���40 �����º��
��β������װ������ͼ��ʾ��˫ͨ�����ܵ����ã� ��
Ũ��������ã� �� �� ����������������
��3��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7825 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000 g������Ʒ�а�������淋����ʵ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����Ӫ���ḻ������������������š����˲����붹��ͬʳ����˵����ijѧУ��ѧ��ȤС���ͬѧ��ͨ��ʵ��̽���������Ƿ��в��������ʣ�����������Щ���ʣ�ͨ��������ѯ������������ϣ����������Ҷ��ᣬ�����Ա�������ǿ�����ἰ���ξ��н�ǿ�Ļ�ԭ�ԣ����ᾧ��(H2C2O4��2H2O)���۵�Ϊ100.1�棬��175��ʱ���ȷֽ⣬�������������ˮ�İ�ɫ���塣
������Ƶ�ʵ�鲽�����£�
1����������������ˮ�����2��3 min����ȴ����ȥ���ˣ�����Һ(�������� ����)������Һ�м�������Ca(OH)2��Һ��Ȼ���ټ�������CH3COOH��Һ���۲�����
����)������Һ�м�������Ca(OH)2��Һ��Ȼ���ټ�������CH3COOH��Һ���۲�����
2���ò��ᾧ��(H2C2O4��2H2O)������ʵ�飺
��ش��������⣺
(1)����1�м���CH3COOH��Һ�����ã� ��
(2)A��Ӧѡ�� (���)������ʵ��֮ǰ��Ӧ�� ��
(3)ʵ��2�����й۲쵽C��Eװ���е���Һ������ǣ���Dװ���к�ɫ��ĩ��Ϊ��ɫ��д��A�в��ᾧ��(H2C2O4��2H2O)������Ӧ�Ļ�ѧ����ʽ�� ��װ��B�������� ��
(4)Ϊʹʵ����۸������ܺͰ�ȫ�������������ӵ�װ��C��D�仹����������װ�� �� �� (Һ���Լ�)��ϴ��ƿ������ָ������װ���еIJ���֮������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���Ȼ��������ظ����(K2Cr2O7)�ζ����ⶨ�Ѿ��������е���������������[w(Fe)]��ʵ�鲽�����£�
����1����ȡ����0.100 g��250 mLϴ������ƿ�С�
����2������FeCl3��Һ100 mL��Ѹ�����Ͻ������õ�Ž���������30 min��
����3�����ˣ���ˮϴ����ƿ��������3��4�Σ�ϴҺȫ��������Һ�С�
����4������Һϡ����500 mL������ȡ100 mLϡ��Һ����ƿ�У�����20 mL���������Ļ���ᣬ��0.5%������������ָʾ��4�Ρ�
����5����K2Cr2O7����Һ�ζ�������������ɫΪ�յ㡣�����ķ�ӦΪCr2O72����6Fe2����14H��=2Cr3����6Fe3����7H2O��
����6���ظ��ⶨ���Ρ�
����7�����ݴ�����
(1)����2�м���FeCl3��Һ����ƿ�з�����Ӧ�����ӷ���ʽΪ________________��Ѹ�����Ͻ�����ԭ����______________________________
(2)����3���ж���ֽ�ϲ�����ϴ���ķ�����_____________________________
(3)ʵ�������õ�100 mLŨ��ԼΪ0.01 mol��L��1K2Cr2O7����Һ������ʱ�õ��IJ���������________________������K2Cr2O7����ǰӦ�Ƚ����������أ���δ��ɣ��Բⶨ�����Ӱ����________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)���ζ�ʱ��K2Cr2O7��ҺӦ����________(����������)�С�
(4)������ԱΪȷ��FeCl3��Һ�����Ũ�ȣ�ѡ��100 mL��ͬŨ�ȵ�FeCl3��Һ(FeCl3��Һ������)�ܽ�ͬһ�Ѿ�����������������������ͬ���õ��ⶨ�����ͼ��ʾ����FeCl3��Һ��Ũ��[�æ�(FeCl3)��ʾ]��ΧӦΪ________g��L��1��Ũ�ȹ��ͻ����ʱ�ⶨ���ƫ�͵�ԭ��ֱ���_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼����A������θҩ������ɿɱ�ʾΪAl2Mg6(OH)x(CO3)y��zH2O��ijУ��ѧ��ȤС�����ⶨ�仯ѧʽ��ʵ��������£�
ʵ��I����ȡһ��������A�����ȷֽ������ء�
ʵ���ȡһ��������A���������ᷴӦ����������CO2�����������
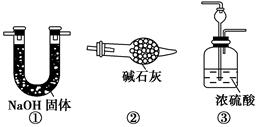
�ɹ�ѡ���������ҩƷ��ͼ��ʾ��������Һ��ѡ6mol/LHCl��6mol/LH2SO4�������Լ���ѡ����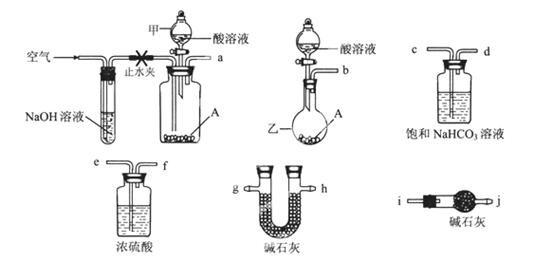
�ش��������⣺
(1)�����ҵ�����Ϊ________��
(2)��ѡ���Ҫ��װ�����ʵ��II,��ȷ������˳��Ϊ________ (�����������ýӿ���ĸ��ʾ����ѡ�õ�����Һ��________��
(3)�������������ʵ��I������ʵ��II��������A��ȫ��Ӧ��������Һ�еμ������İ�ˮ��������ֽ���ˣ�������ˮϴ�ӷ�Ӧ����2?3�Σ���ϴ��Һ���ˣ�ϴ�ӳ���2?3�Σ������ų�������ֽ�ŵ������м��ȷֽ������ء��жϳ�����ϴ�Ӹɾ��ķ�����_________________,ʵ����δ���ø÷�����ԭ���Dz�����ʵ����Ƶ�________ԭ������ĸ��ţ���
| A����ѧ�� | B����ȫ�� | C�������� | D����Լ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��Ϊ�ⶨ�ӵ�����KIO3����������������������ʵ�顣
��֪��KIO3 + 5KI + 3H2SO4�� 3K2SO4 + 3I2 + 3H2O
I2 + 2Na2S2O3 �� Na2S4O6 + 2NaI
����һ��ȷ��ȡa g�ӵ��Σ����Ƴ�250mL��Һ��
�������ȡ��������Һ25.00mL����ƿ�У���ϡ�����ữ���ټ���������KI��Һ��
����������bmol��L��1 Na2S2O3��Һ����Һ�ζ������������Һ���յ㣬��¼���ݣ����ظ��ζ�2�Σ�ƽ������Na2S2O3��Һ�����Ϊ12.00mL��
��1������һ������250mL��Һ�����õ��IJ����������ձ����������ͽ�ͷ�ι��⣬���� ��
��2���������н��еζ��Ĺ����������ĸ�������ȷ ��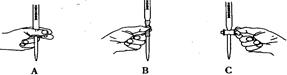
ѡ�� ��Ϊָʾ��������ζ��յ�ʱ������Ϊ ��
��3��ʵ���ô˼ӵ�����KIO3������������ ��KIO3����Է�������Ϊ214����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��С���������о��¶ȶԷ�Ӧ���ʵ�Ӱ�족ʵ��ʱ����ȡ����ֻ�Թܣ�������4mL 0.01mol��L��KMnO4������Һ��2mL 0.1mol/L H2C2O4���Ҷ��ᣩ��Һ����A�Թ�������ˮ�У�B�Թ�������ˮ�У���¼��Һ��ɫ�����ʱ�䡣
����Ҫ�� ���ữKMnO4��Һ����ɫ����ʱ��tA tB���>������=����<������
��д���÷�Ӧ�����ӷ���ʽ ��
��2��ʵ������ƿ������ɳ���Ҷ�����Ʒ��С�����������Ӧ��ԭ�����ⶨ�京�����������Ϊ��
������250 mL��Һ��ȷ����5.0g�Ҷ�����Ʒ�����250mL��Һ��
�ڵζ���ȷ��ȡ25.00 mL������Һ����ƿ�У����������ữ����0.1000 mol��L��1 KMnO4��Һװ�� �����ʽ����ʽ�����ζ��ܣ����еζ�������
��ʵ���з��֣��յ�������KMnO4��Һʱ����ҺѸ�ٱ���Ϻ�ɫ������ƿҡ��һ��ʱ����Ϻ�ɫ������ʧ���ټ����μ�ʱ���Ϻ�ɫ�ͺܿ���ɫ�ˡ������ԭ��
����____
��֤���ﵽ�ζ��յ㡣
�ۼ��㣺���ظ���������2�Σ���¼ʵ���������¡�
| ��� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 0.00 | 20.10 |
| 2 | 1.00 | 20.90 |
| 3 | 1.10 | 21.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ�ã������豸���г̶ֹ�װ�þ���ȥ���������Ҫ��������и��⣨����װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ�ᡢ��Ϊ��������
��1�����������ͨ��CO��CO2�Ļ�����壬���ڷ���CuO��ѡ��װ�û�ô��������CO������֤�仹ԭ�Լ����������ѡװ�õ�����˳��Ϊ������������������ţ�������֤CO���������������������������������������
��2��ֹͣCO��CO2��������ͨ�룬���ڷ���Na2O2����A��E��D��B��Hװ��˳����ȡ���������O2������O2�����Ҵ�����ʱ������aӦ����������������bӦ����������Ҫ���ȵ�����װ��������������������ţ������з�Ӧ�Ļ�ѧ����ʽΪ����������������
��3����������ڸ�ͨ��������Һ©���ڸļ�Ũ��ˮ��Բ����ƿ�ڸļ�NaOH���壬���ڷ��ò���Ͻ�������A��G��E��Dװ��˳����ȡ����İ���������֤����ijЩ���ʡ�
��װ��A���ܲ���������ԭ��������������������������
��ʵ���й۲쵽E���к���ɫ������֣�֤�������������������ԡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com