
[ѡ��2������ѧ�뼼��] ��15�֣�
I����ˮ����һ����������������������Cu2+��Hg2+��Pb2+���ؽ������ӣ��ɼ��������ʹ���������������������Ϊ����������
A����ˮ B���������� C����������Һ D��������Һ
II���ϳɰ�������ʾ��ͼ���£�
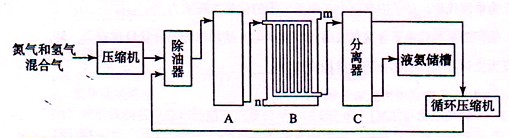
�ش��������⣺
(1)��ҵ�ϳɰ���ԭ���ǵ����������������Ǵӿ����з�������ģ�ͨ��ʹ�õ����ַ��뷽���� �� ����������Դ��ˮ��̼�⻯���д���ֱ����ú����Ȼ��Ϊԭ����ȡ�����Ļ�ѧ��Ӧ����ʽ ��
��
(2) �豸A�к��е����������ú���Ƚ��������豸A������ �����з����Ļ�ѧ��Ӧ����ʽΪ ��
(3) �豸B������ ������m��n������ͨˮ�ڣ���ˮ���� ���m����n���������˴��෴����ͨˮ��ԭ�� ��
(4) �豸C������ ��
(5)��ԭ�����Ʊ������л���CO�Դ����ж������ã�����ȥԭ�����е�CO����ͨ�����·�Ӧ��ʵ�֣�CO(g)+H2O(g) 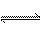 CO2 (g)+ H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ ��
CO2 (g)+ H2 (g) �� ��֪1000Kʱ�÷�Ӧ��ƽ�ⳣ��K=0.627����ҪʹCO��ת������90%������ʼ����c(H2O)��c(CO)������ ��
 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(2013���������ۣ�12)��ʡʢ������(��Ҫ�ɷ���NaCl��������SO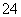 ���������������ʵ�����)�������й�˵����ȷ
���������������ʵ�����)�������й�˵����ȷ ���� (����)
���� (����)
A���ɿ�������ʳ�Σ���ȥSO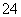 ����ʵ��Լ���Ba(NO3)2
����ʵ��Լ���Ba(NO3)2
B����ҵ��ͨ������Ȼ�����Һ�Ʊ������ƺ�����
C�������£�AgCl��ˮ�е��ܽ��С����ʳ��ˮ�е��ܽ��
D���÷�̪��Һ�ɼ��𱥺�ʳ��ˮ�ͱ��ʹ�����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ϩ�Ʋ��ϩ����ˮ��Ӧʱ���Է�Ӧ�����������ȷ����(����)
A��CH2Br��CH2��CH2Br B��CH3��CHBr��CH3
C��CH3��CH2��CHBr2 D��CH3��CHBr��CH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
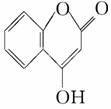 һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ��
һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ��
��ͨ������·�ߺϳɣ�
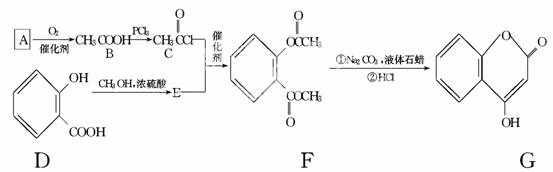
��1��A��������Һ��Ӧ���������ɣ���A�Ľṹ��ʽ��____________________��
��2��B��C�ķ�Ӧ������________________________��
��3��E�Ľṹ��ʽ��________________________��
��4��д��F����NaOH��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��
____________________________________________________________________.
��5�����й���G��˵����ȷ����________��
a�������嵥�ʷ�Ӧ
b����������Ʒ�Ӧ
c��1 mol G����ܺ�3 mol������Ӧ
d������ʽ��C9H6O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϡ���ͼ��ͨ���˹���������Ʊ�HCOOH��ԭ��ʾ��ͼ������˵������ȷ����
A���ù����ǽ�̫����ת��Ϊ��ѧ�ܵĹ���
B������a���淢��������Ӧ����O2����
C������a�������Լ���������b����������ǿ
D������b����ķ�Ӧ��CO2 +2H++2eһ= HCOOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)____S��____KOH===____K2S��____K2SO3��____H2O
(2)____P4��____KOH��____H2O===____K3PO4��____PH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡx gͭþ�Ͻ���ȫ����Ũ�����У���Ӧ���������ᱻ��ԭֻ����8 960 mL��NO2�����672 mL��N2O4����(�������㵽��״̬)���ڷ�Ӧ�����Һ�м�������������������Һ�����ɳ�������Ϊ17.02 g����x���� (����)
A��8.64 B��9.20
C��9.00 D��9.44
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
a mol Cu�뺬b mol HNO3����Һǡ����ȫ��Ӧ������ԭ��HNO3�����ʵ���һ����(����)
A��(b��2 a) mol B.
a) mol B.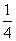 b mol
b mol
C.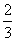 a mol D��2a mol
a mol D��2a mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�������ֵ������˵����ȷ���� �� ��
A��100mL 2 mol��L-1̼������Һ�У�CO32-������Ϊ0.2NA
B����10mL 1mol��L-1FeCl3��Һ�����ˮ�У�������������������Ϊ0.01NA
C��1 mol Na2O2�����к���������Ϊ3NA
D����⾫��ͭʱ�����·��ת�Ƶ�����Ϊ2NA��ʱ������ͭ��������64g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com