
·ÖĪö »īĘĆ½šŹōŗĶ»īĘĆ·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³ÉĄė×Ó¼ü£¬²»Ķ¬·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³É¼«ŠŌ¼ü£¬Ķ¬ÖÖ·Ē½šŹōŌŖĖŲÖ®¼äŅ׊Ī³É·Ē¼«ŠŌ¼ü£¬ŗ¬ÓŠ¹Āµē×Ó¶ŌŗĶŗ¬ÓŠæÕ¹ģµĄµÄŌ×ÓÖ®¼äŅ׊Ī³ÉÅäĪ»¼ü£»ÓÉŅõŃōĄė×Ó¹²“ęµÄ¾§ĢåŹĒĄė×Ó¾§Ģ壬ÓÉ·Ö×Ó¹¹³ÉµÄ¾§ĢåŹĒ·Ö×Ó¾§Ģ壬ÓÉŌ×Ó¹¹³ÉµÄ¾§ĢåŹĒŌ×Ó¾§Ģå£¬ÕżøŗµēŗÉÖŲŠÄÖŲŗĻµÄ·Ö×ÓŹĒ·Ē¼«ŠŌ·Ö×Ó£¬ÕżøŗµēŗÉÖŲŠÄ²»ÖŲŗĻµÄ·Ö×ÓŹĒ¼«ŠŌ·Ö×Ó£¬¾Ż“Ė·ÖĪö½ā“š£®
½ā“š ½ā£ŗ¢ŁNaOHÖŠÄĘĄė×ÓŗĶĒāŃõøłĄė×ÓÖ®¼ä“ęŌŚĄė×Ó¼ü”¢H-OŌ×ÓÖ®¼ä“ęŌŚ¼«ŠŌ¼ü£¬ĪŖŗ¬ÓŠĄė×Ó¼üŗĶ¼«ŠŌ¼üµÄĄė×Ó¾§Ģ壻
¢ŚNa2SÖŠÖ»ŗ¬Ąė×Ó¼ü£¬ĪŖĄė×Ó¾§Ģ壻
¢ŪSiCŹĒÓÉŌ×Ó¹¹³ÉµÄŌ×Ó¾§Ģ壬Si”¢CŌ×ÓÖ®¼äÖ»“ęŌŚ¼«ŠŌ¼ü£»
¢ÜC2H2ÖŠC-CŌ×ÓÖ®¼ä“ęŌŚ·Ē¼«ŠŌ¼ü”¢C-HŌ×ÓÖ®¼ä“ęŌŚ¼«ŠŌ¼ü£¬ÕżøŗµēŗÉÖŲŠÄÖŲŗĻ£¬ĖłŅŌŹĒ·Ē¼«ŠŌ¼ü·Ö×Ó£¬ŹōÓŚ·Ö×Ó¾§Ģ壻
¢ŻNa2O2ÖŠÄĘĄė×ÓŗĶ¹żŃõøłĄė×ÓÖ®¼ä“ęŌŚĄė×Ó¼ü£¬O-OŌ×ÓÖ®¼ä“ęŌŚ·Ē¼«ŠŌ¼ü£¬ĪŖĄė×Ó¾§Ģ壻
¢Ž£ØNH4£©2SÖŠĮņĄė×ÓŗĶļ§øłĄė×ÓÖ®¼ä“ęŌŚĄė×Ó¼ü”¢N-HŌ×ÓÖ®¼ä“ęŌŚ¼«ŠŌ¼üĒŅÓŠŅ»øöŹĒÅäĪ»¼ü£¬ĪŖĄė×Ó¾§Ģ壻
£Ø1£©ĘäÖŠÖ»ŗ¬ÓŠĄė×Ó¼üµÄĄė×Ó¾§ĢåŹĒ¢Ś£¬¹Ź“š°øĪŖ£ŗ¢Ś£»
£Ø2£©ĘäÖŠ¼Čŗ¬ÓŠĄė×Ó¼üÓÖŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üµÄĄė×Ó¾§ĢåŹĒ¢Ł”¢¢Ž£¬¹Ź“š°øĪŖ£ŗ¢Ł”¢¢Ž£»
£Ø3£©ĘäÖŠ¼Čŗ¬ÓŠĄė×Ó¼ü£¬ÓÖŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üŗĶÅäĪ»¼üµÄĄė×Ó¾§ĢåŹĒ¢Ž£¬¹Ź“š°øĪŖ£ŗ¢Ž£»
£Ø4£©ĘäÖŠ¼Čŗ¬ÓŠĄė×Ó¼üÓÖŗ¬ÓŠ·Ē¼«ŠŌ¹²¼Ū¼üµÄĄė×Ó¾§ĢåŹĒ¢Ż£¬¹Ź“š°øĪŖ£ŗ¢Ż£»
£Ø5£©ĘäÖŠŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üŗĶ·Ē¼«ŠŌ¹²¼Ū¼üµÄ·Ē¼«ŠŌ·Ö×ÓŹĒ¢Ü£¬¹Ź“š°øĪŖ£ŗ¢Ü£»
£Ø6£©ĘäÖŠŗ¬ÓŠ¼«ŠŌ¹²¼Ū¼üµÄŌ×Ó¾§ĢåŹĒ¢Ū£¬¹Ź“š°øĪŖ£ŗ¢Ū£®
µćĘĄ ±¾Ģāæ¼²é»Æѧ¼ü£¬ĪŖøßĘµæ¼µć£¬Ć÷Č·Ąė×Ó¼ü¹²¼Ū¼üĒų±šŹĒ½ā±¾Ģā¹Ų¼ü£¬²ąÖŲæ¼²é»ł±¾øÅÄī£¬×¢Ņā£ŗÅäĪ»¼üŹōÓŚ¹²¼Ū¼ü£¬ĪŖŅדķµć£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Z | M | R | Q | |
| Ō×Ó°ė¾¶/nm | 0.186 | 0.074 | 0.099 | 0.143 |
| Ö÷ŅŖ»ÆŗĻ¼Ū | -2 | -1£¬+7 | +3 |
| A£® | XÓėMŠĪ³ÉµÄ»ÆŗĻĪļÖŠŗ¬ÓŠĄė×Ó¼ü | |
| B£® | Z”¢M”¢QČżÖÖŌŖĖŲµÄ¼ņµ„Ąė×ӵİė¾¶£ŗM£¾Z£¾Q | |
| C£® | YÓėRŠĪ³ÉµÄ»ÆŗĻĪļÖŠR³ŹÕż¼Ū£¬ĖµĆ÷YµÄ·Ē½šŹōŠŌ±ČRĒæ | |
| D£® | ŌŚŌŖĖŲÖÜĘŚ±ķÖŠ£¬QĪ»ÓŚ½šŹōÓė·Ē½šŹōµÄ½»½ē“¦£¬æÉŅŌ×÷°ėµ¼Ģå²ÄĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ź×¼×“æöĻĀ£¬22.4 L µÄCCl4ŗ¬ÓŠ4NAøöClŌ×Ó | |
| B£® | 0.1 mol•L-1Na2SČÜŅŗÖŠŗ¬ÓŠ0.1NAøöS2- | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬92gµÄNO2ŗĶN2O4»ģŗĻĘųĢåŗ¬ÓŠµÄNŌ×ÓŹżĪŖ2NA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬22.4LĀČĘųÓė×ćĮæµÄĢś·Ū·“Ó¦£¬×ŖŅʵĵē×ÓŹżĪŖ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ķŹ¾ĪļÖŹŹżĮæµÄµ„Ī» | |
| B£® | ÓĆ¾Ž“óŹżĮæµÄĪ¢Į£¼ÆĢå±ķŹ¾ĪļÖŹµÄĮæµÄµ„Ī» | |
| C£® | ±ķŹ¾ĪļÖŹµÄÖŹĮ浄Ī» | |
| D£® | ¼Č±ķŹ¾ĪļÖŹµÄÖŹĮæÓÖ±ķŹ¾ĪļÖŹĪ¢Į£µÄŹżĮ浄Ī» |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1molCl2²Ī¼Ó»Æѧ·“Ó¦»ńµĆµÄµē×ÓŹż¶¼ŹĒ2NA | |
| B£® | 6.4 gO2ÓėO3µÄ»ģŗĻĪļÖŠĖłŗ¬OŌ×ÓŹżŅ»¶ØĪŖ0.4NA | |
| C£® | ŗ¬4nmol HClµÄÅØŃĪĖįÓė×ćĮæµÄMnO2¼ÓČČ·“Ó¦£¬Éś³ÉµÄCl2·Ö×ÓŹżĪŖnNA | |
| D£® | 1molijĘųĢåµÄĢå»żĪŖ22.4L£¬øĆĘųĢåµÄדæöŅ»¶ØŹĒ±ź×¼×“æö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
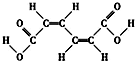
| A£® | øĆĪļÖŹÓŠČżÖÖ3ÖÖ²»Ķ¬»Æѧ»·¾³µÄĒā£Ø²»æ¼ĀĒĖ³·“Ņģ¹¹£© | |
| B£® | 1moløĆÓŠ»śĪļæÉŅŌŗĶ2mol NaOH·“Ó¦£¬µ«²»ÄÜÓĆNa2CO3ČÜŅŗĒų·ÖøĆÓŠ»śĪļŗĶCH3COOCH2CH3 | |
| C£® | øĆÓŠ»śĪļÄÜ·¢Éś¼Ó³É·“Ó¦²»ÄÜ·¢ÉśČ”“ś·“Ó¦ | |
| D£® | øĆÓŠ»śĪļÓėŅŅĖį»„ĪŖĶ¬ĻµĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓŠ»śĪļ¶¼ÄÜČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®£¬ĒŅŹÜČČ²»Ņ×·Ö½ā | |
| B£® | ÓŠ»śĪļ“󶹏żÄŃČÜÓŚĖ®¶ųŅ×ČÜÓŚĘū”¢ĖÄĀČ»ÆĢ¼µČÓŠ»śČܼĮ | |
| C£® | ÓŠ»śĪļ¶¼ŹĒ·Ēµē½āÖŹĒŅČŪµć”¢·Šµć½ĻµĶ | |
| D£® | ÓŠ»ś»Æѧ·“Ó¦¶¼½Ļø“ŌÓ£¬ĒŅø±·“Ó¦¶ą£¬·“Ó¦ĖŁĀŹĀż |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com