
����Ŀ��B��N��Ti��Fe������Ҫ�IJ���Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
��1����̬Fe2���ĵ����Ų�ʽΪ_____��Tiԭ�Ӻ����________���˶�״̬��ͬ�ĵ��ӡ�
��2��BH3������NH3���ӵĿռ�ṹ�ֱ�Ϊ_________��BH3��NH3��Ӧ���ɵ�BH3��NH3�����к��еĻ�ѧ��������_______����BH3��NH3��Bԭ�ӵ��ӻ���ʽΪ________��
��3��N��Pͬ���塣��ѧ��Ŀǰ�ϳ���N4���ӣ��÷�����N��N��N���ļ���Ϊ________��N4�ֽ���ܲ���N2���ͷų������������Ʋ�����;___________��(д��һ�ּ���)
��4��NH3��Cu2�����γ�[Cu(NH3)4]2�������ӡ���֪NF3��NH3������ͬ�Ŀռ乹�ͣ���NF3������Cu2���γ������ӣ���ԭ����____��
��5������TiO2��һ��Ӧ�ù㷺�Ĵ����������һ��ʵ����ͼ��ʾ���������ҵķе����Ը��ڻ�����ף���Ҫԭ����______�����������в�ȡsp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________��

���𰸡�1s2 2s2 2p6 3s2 3p6 3d6 ��[Ar]3d6 22 ƽ���������Ρ������� ���ۼ�����λ�� sp3 60�� �������ƽ�����ըҩ(���������𰸾���) F �ĵ縺�Ա� N ��N��F �ɼ����Ӷ�ƫ�� F������ NF3 �е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ�� �������ҷ��Ӽ������� N>O>C
��������
(1)��Ϊ26��Ԫ�أ�ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+���ݴ���д��������Ų�ʽ�������ÿ�����ӵ��˶�״̬����ͬ��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
(3)N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�ÿ�����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų������������ݴ��ж���;��
(4)NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�
(5)����Ĵ��ڵ��������۷е����ߣ��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ��ݴ�����
(1)��ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d6��[Ar]3d6��TiԪ��ԭ�Ӻ�����22�����ӣ�����ԭ�����˶�״̬��ͬ�ĵ��ӹ���22�֣��ʴ�Ϊ��1s22s22p63s23p63d6��[Ar]3d6��22��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�BF3��Bԭ�Ӻ���3�������Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�NH3��Nԭ�Ӻ���3��������1���µ��Ӷԣ�����NH3Ϊ�������ͣ�BF3NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3NH3��Bԭ�ӵļ۲���Ӷ���Ϊ4+0=4���ӻ���ʽΪsp3���ʴ�Ϊ��ƽ���������Σ������ͣ����ۼ�����λ����sp3��
(3)N4������Nԭ���γ�3������������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60����N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ���ʴ�Ϊ��60�����������ƽ�����ըҩ��
(4)F�ĵ縺�Դ���NԪ�أ�NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�����NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ��Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�����NF3������Cu2+�γ������ӣ��ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
(5)����Ĵ��ڻᵼ�������۷е����ߣ����к��з��Ӽ�������ײ�����������Ի��������۷е���ڼף��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������N��O��C���ʴ�Ϊ���������ҷ��Ӽ���������N��O��C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ����
A. ���³�ѹ�£�124 g P4������P��P����ĿΪ4NA
B. 100 mL 1mol��L1FeCl3��Һ������Fe3+����ĿΪ0.1NA
C. ��״���£�11.2 L�������ϩ������к���ԭ����ĿΪ2NA
D. �ܱ������У�2 mol SO2��1 mol O2����Ӧ���������Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ����2 L���ܱ������У�X��Y��Z �������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ��
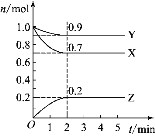
(1)��ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽ_____________��
(2)�ӷ�Ӧ��ʼ��2 min��Z��ƽ����Ӧ����Ϊ________��
(3)��5 minʱ��Z����������________(����ڡ���С�ڡ����ڡ�)Z���������ʡ�
(4)��_______����ʱ����Ӧ�ﵽƽ�⡣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ǻϳ����ᡢ��κ͵��ʵĻ���ԭ�ϣ��ش��������⣺
��1��NH3�ĵ���ʽ______��
��2���Ȼ��ˮ��Һ�����ԣ���ԭ��Ϊ______�������ӷ���ʽ��ʾ����0.1mol/L�İ�ˮ�м���������NH4Cl���壬��Һ��pH______������������������������������������������������볢�Դ�ƽ���ƶ��ĽǶȽ�����Һ��NH4+Ũ�ȵı仯ԭ��______��
��3������識��ȷֽ�ɵõ�N2O��g����H2O��g����250��ʱ����������ܱ������зֽ�ﵽƽ�⣬���¶��·�Ӧ��ƽ�ⳣ������ʽΪ______������1mol�������ȫ�ֽ⣬ת�Ƶĵ�����Ϊ______mol��
��4��3H2��g��+N2��g��2NH3��g����H=-92kJ/mol������Ӧ�ų�9.2kJ ��������μӷ�Ӧ������������ĿΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
��1�����Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��������������Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣��д�����з�Ӧ�Ļ�ѧ����ʽ______��
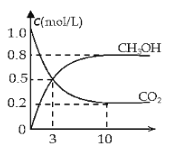
��2���罫CO2��H2��1��3������Ȼ�ϣ����ʵ������ºϳ�ȼ�ϼ״���ˮ�������Ϊ2L���ܱ������У�����2mol CO2��6mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��g����CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
�ӷ�Ӧ��ʼ��ƽ�⣬v��H2��=______��������ת����=______����ʹƽ����ϵ��n��CH3OH������Ĵ�ʩ��______����ֻдһ�ּ��ɣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����г����¼ס��ҡ���������Һ����Ϊ0.1 mol��L��1��NaOH��Һ����Ϊ0.1 mol��L��1��HCl��Һ����Ϊ0.1 mol��L��1��CH3COOH��Һ���Իش��������⣺
��1������Һ��pH��________��
��2������Һ�д��ڵĵ���ƽ��Ϊ______________(�õ���ƽ�ⷽ��ʽ��ʾ)��
��3�������£���ˮϡ��0.1 mol��L��1��CH3COOH��Һʱ�����и�����ˮ�������Ӷ��������________(�����)��
��n(H��)������������c(H��) �� c(CH3COOH)/c(CH3COO-) ��c(OH��)
��4���ס��ҡ���������Һ����ˮ�������c(OH��)�Ĵ�С��ϵΪ___________��
��5��ijͬѧ�ü���Һ�ֱ�ζ�20.00 mL����Һ��20.00 mL����Һ���õ���ͼ��ʾ�������ζ����ߣ���ش��й����⣺
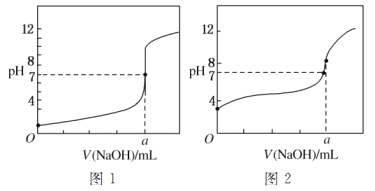
�ټ���Һ�ζ�����Һ��������________(����ͼ1������ͼ2��)���ߡ�
��a��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. 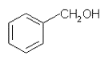 ��
��![]() ������ͬ�Ĺ����ţ���Ϊͬϵ��
������ͬ�Ĺ����ţ���Ϊͬϵ��
B.  ����ȩ�࣬������Ϊ��CHO
����ȩ�࣬������Ϊ��CHO
C. ![]() ������Ϊ��2���һ���1����ϩ
������Ϊ��2���һ���1����ϩ
D. ![]() ������Ϊ��2������1��3������ϩ
������Ϊ��2������1��3������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҵ�����Ҫ���л�����ԭ�ϣ�Ҳ�����ʵ�ȼ�ϣ���ҵ�Ͽ�����ϩˮ�Ϸ��ͷ��������ش��������⣺
��1����ϩˮ�Ϸ��ɷ�Ϊ����
��һ������ӦCH2��CH2+ HOSO3H��Ũ���ᣩ��CH3CH2OSO3H����������������
�ڶ���������������ˮ�������Ҵ���
�ٵ�һ�����ڷ�Ӧ_______________���Ӧ���ͣ���
�ڵڶ�����Ӧ�Ļ�ѧ����ʽΪ_____________________________��
��2�����ͷ����Ҵ���ֲ��ոѣ���50����ά�أ�Ϊԭ�Ͼ�����ת���Ƶ��Ҵ�
��ά�صĻ�ѧʽΪ________����Ҫ��ȡ4.6 ���Ҵ���������Ҫֲ��ո�________�֡�
��3���Ҵ���������90������ͨ������10����ȼ���Ҵ����Ͷ��ɡ��Ҵ��������Ҵ��ǿ�������Դ����Դ��________�����ϩˮ�Ϸ������ͷ�������
��4�����Ҵ�Ϊԭ�Ͽ��Ʊ�ij�ָ߷���Ϳ�ϣ���ת����ϵ����ͼ��

���л���A�Ľṹ��ʽΪ___________��
�ڷ�Ӧ��Ļ�ѧ����ʽΪ___________��
�۷�Ӧ��ķ�Ӧ����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ԷϾ�����ӵ�ص��������ϣ���Ҫ��LiCoO2��Al��C�ȣ�Ϊԭ���Ʊ�CoC2O4.2H2O��һ��ʵ��������ͼ��
![]()
![]()
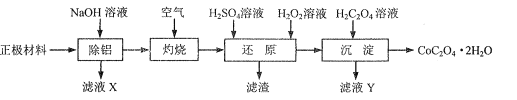
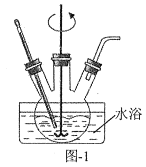
��1����������������ͼ1��ʾ��װ���н��С������¶ȡ���Ӧ����ܼ��������䣬ʵ�����������ȥ���ʵĴ�ʩ��___��
��2��������������ҪĿ����___��
��3������ԭ�������¶���70�����ң�LiCoO2������Ӧ�Ļ�ѧ����ʽΪ___�����ò������������H2SO4��H2O2��Ҳ�ɴﵽ����ԭ����Ŀ�ģ�����ȱ����___��
��4���������������У�֤��Co2+�ѳ�����ȫ��ʵ�������������___��
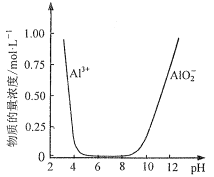
��5�����������ҺX���Ʊ�������Al2O3��ʵ�鷽��������֪��������Ũ����pH�Ĺ�ϵ��ͼ��ʾ��ʵ���б���ʹ�õ��Լ���H2SO4��Һ��BaCl2��Һ������ˮ��___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com