
 ��
��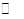 Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������
Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

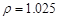 g/cm3 )����ȡ��36.5% (
g/cm3 )����ȡ��36.5% (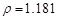 g/cm3 )������ mL
g/cm3 )������ mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
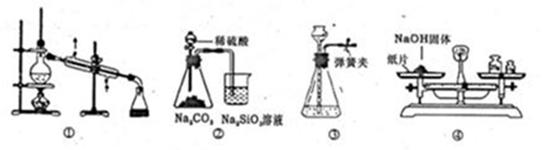
| A��װ�âٿ������Ҵ���ˮ�ķ��� |
| B��װ�â���ʾʵ��ɱȽ���̼��������Ԫ�صķǽ�����ǿ�� |
| C��װ�âۣ��رյ��ɼУ���©����עˮ����©����ˮ��߶ȱ��ֲ�����˵��װ�ò�©�� |
| D��ͼ��װ�ÿ���������һ��������NaOH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ж�������Ӧ�Ƿ���ȫ����ȡ��Ӧ��Ļ��Һ������ˮ�� |
| B����ѹ���˲��˹��˽�״���������̫С�ij��� |
| C���Ʊ���������茶��壬���������������������ʣ������Һ�弴ֹͣ���� |
| D����ȼ�Լ���ǿ�������Լ���ֿ����ã���Զ���Դ�������Ż�ʱ.����ϸɳ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��ij�������ȵμ�ϡ�����ữ���ٵμ�BaCl2��Һ���а�ɫ�������� | ����Һ��һ������Ag�� |
| B | �������ͨ�����͵�Na2CO3��Һ | ��ȥCO2�л��е�HCl |
| C | ����SnCl2��Һʱ���Ƚ�SnCl2����������ϡ�����У���������ˮϡ�ͣ�����ʱ���Լ�ƿ�м������������� | ����Sn2��ˮ�⣬����ֹSn2��������ΪSn4�� |
| D | ����������ˮ�У������ã���ˮ����ɫ | ������ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
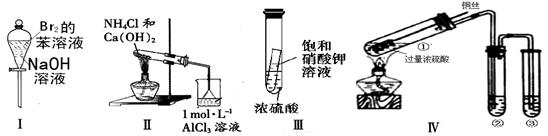
| A��ʵ��I�����ã��²���Һ��ɫ���� |
| B��ʵ��� ���ձ����ȳ��ְ�ɫ���������ܽ� |
| C��ʵ��III������һ��ʱ���С�Թ����о������� |
| D��ʵ�����Ϊȷ��CuSO4���ɣ�����м�ˮ���۲���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ȡ����Ӧ | B������������Ӧ | C��������������ȡ | D��ʯ�͵ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
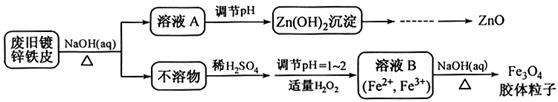
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com