
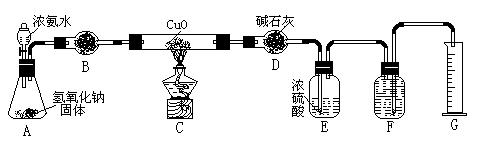
| A����ʯ�� | B����ʯ�� | C������������ | D���������� |
 5����ʵ����Ϻ�,��ʵ����N2�����(����ɱ�״��)a L,�����D����b g, �����е������ԭ�Ӹ�����Ϊ���ú�a��b��ĸ�Ĵ���ʽ��ʾ�� ��
5����ʵ����Ϻ�,��ʵ����N2�����(����ɱ�״��)a L,�����D����b g, �����е������ԭ�Ӹ�����Ϊ���ú�a��b��ĸ�Ĵ���ʽ��ʾ�� ��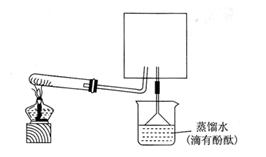
 ��
��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
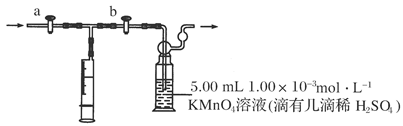
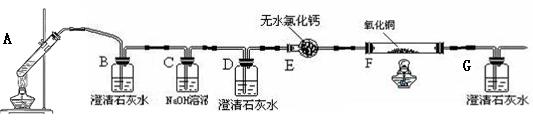 ��1��װ��C�������� _______��װ��E�������� ��
��1��װ��C�������� _______��װ��E�������� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
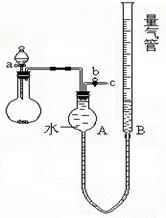
| | ���ʹ������� | �����Ƶ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| �� | 0.62 g | 5 .0g(����) | 40 mL | 264 mL |
| �� | 0.31 g | 2.5 g(����) | 40 mL | 152mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��ʦ�ڿ�������ʾ������ʵ��װ�ã�����̨�ȸ���������ȥδ������
��ʦ�ڿ�������ʾ������ʵ��װ�ã�����̨�ȸ���������ȥδ������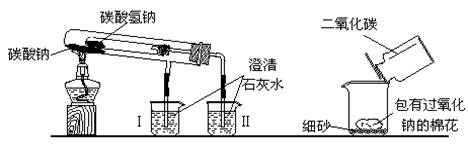
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
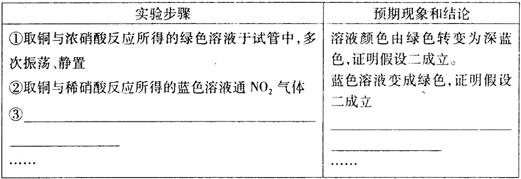
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| | ѡ���Լ� | ʵ������ |
| ��һ�ַ��� | | |
| �ڶ��ַ��� | | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com