



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 H++OH-,��25 ��ʱˮ�����ӻ�ΪKW=1.0��10-14,��35 ��ʱˮ�����ӻ�ΪKW=2.1��10-14,������������ȷ����(����)
H++OH-,��25 ��ʱˮ�����ӻ�ΪKW=1.0��10-14,��35 ��ʱˮ�����ӻ�ΪKW=2.1��10-14,������������ȷ����(����)| A��c(H+)�����¶ȵ����߶����� |
| B��35 ��ʱc(H+)>c(OH-) |
| C��35 ��ʱ��ˮ��25 ��ʱ��ˮ����̶�С |
| D��ˮ�ĵ����Ǹ����ȹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��c(Na��)��2c(CO32��) |
| B��c(CO32��)��c(OH��)��c(HCO3��)��c(H2CO3) |
| C��c(OH��)��c(H��)��c(HCO3��)��2c(H2CO3) |
| D��c(CO32��)��c(HCO3��)="0.1" mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��ͼ�ж�Ӧ����¶ȹ�ϵΪA>B |
| B��ˮ�ĵ��볣��KW��ֵ��С��ϵΪB>D |
| C���¶Ȳ��䣬��������NaOH��ʹ��Һ��C��䵽A�� |
| D����B���Ӧ�¶��£���pH��2��H2SO4��pH��10��NaOH��Һ�������Ϻ���Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H2O(g)=H2(g)��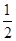 O2(g)����H����242 kJ��mol��1 O2(g)����H����242 kJ��mol��1 |
| B��2H2(g)��O2(g)=2H2O(l)����H����484 kJ��mol��1 |
C��H2(g)��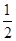 O2(g)=H2O(g)����H����242 kJ��mol��1 O2(g)=H2O(g)����H����242 kJ��mol��1 |
| D��2H2(g)��O2(g)=2H2O(g)����H����484 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��̫���ܡ������ܡ��������ܺͺ˾۱��ܾ����ڡ�����Դ�� |
| B������̼����ָ���ú�̼���͵�������Ϊȼ�� |
C����ͼ���龭һ�ȼ������ɵ�̼ϩ����;�������ˡ����ܼ��š�˼�� |
| D����ú��ɺϳ�������ú���Ը�ե������ʵ����ú����ࡢ��Ч���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com