
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺���� ��1������Һ���ʵ���Ũ��=$\frac{1{0}^{3}�Ѧ�}{M}$��
��2����Һ�ܶȡ����ʵ���Ũ������Һ����أ���Һ���������ʵ�����������������йأ�
��3���ٸ�����Һϡ��ǰ�����ʵ����ʵ����������Ũ�����������Ͳ���Ӧ���Դ��ڻ����Ũ���������
���ý�ͷ�ιܶ��ݣ���ƽ��ʹ��Һ����ǡ����̶�������Ϊֹ��
�۸���c=$\frac{n}{V}$�ж������nƫС��Vƫ��ᵼ��������ҺŨ��ƫ�ͣ����nƫ���VƫС��������ҺŨ��ƫ�ߣ�
��4�������Һ��c��SO42-��=$\frac{n��{H}_{2}S{O}_{4}��+n��CuS{O}_{4}��}{V�������Һ��}$��
��� �⣺��1������Һ���ʵ���Ũ��=$\frac{1{0}^{3}�Ѧ�}{M}$=$\frac{1{0}^{3}��1.84��98%}{98}$mol/L=18.4mol/L��
�ʴ�Ϊ��18.4��
��2��A������n=cV֪�����ʵ����ʵ�������Һ����йأ���A��ѡ��
B����Һ�Ǿ�һ�ȶ��ģ����ʵ���Ũ������Һ����أ���Bѡ��
C������N=cVNA֪����Һ����������Ӹ�������Һ����йأ���C��ѡ��
D����Һ�Ǿ�һ�ȶ��ģ��ܶ�����Һ����أ���Dѡ��
��ѡBD��
��3������Һϡ��ǰ�����ʵ����ʵ������䣬��Ũ�������=$\frac{0.2mol/L��0.5L}{18.4mol/L}$=5.4mL��
�ʴ�Ϊ��5.4��
�ڲ���EΪ���ý�ͷ�ιܶ��ݣ���ƽ��ʹ��Һ����ǡ����̶�������Ϊֹ��
�ʴ�Ϊ�����ý�ͷ�ιܼ�ˮ��ƽ��ʹ��Һ����ǡ����̶������У�
��A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������Һ���ƫ�����ʵ����ʵ���ƫ������������ҺŨ��ƫ�ߣ�����ȷ��
B��ϡ���õ��ձ��Ͳ�����δϴ�ӣ��������ʵ����ʵ���ƫС����������ҺŨ��ƫ�ͣ��ʴ���
C��ϴ��������ƿδ�����������������Һ�����ʵ����ʵ������䡢��Һ������䣬��������ҺŨ����Ӱ�죬�ʴ���
D����ˮ�����̶��ߺ��ý�ͷ�ι����������Һ�壬���ʵ����ʵ���ƫС��������ҺŨ��ƫ�ͣ��ʴ���
��ѡA��
��4�������Һ��c��SO42-��=$\frac{n��{H}_{2}S{O}_{4}��+n��CuS{O}_{4}��}{V�������Һ��}$=$\frac{0.2mol/L��0.1L+0.4mol/L��0.3L}{��0.1+0.3��L}$=0.35mol/L��
�ʴ�Ϊ��0.35��
���� ���⿼��һ�����ʵ���Ũ����Һ���ƣ����ؿ���ѧ��ʵ�����������������������ȷ�������衢�����������ʵ���Ũ�ȼ��㷽�����ɽ���״�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ������Ҫ�ɷ�����ϩ | |
| B�� | ú���ͷ�����Ի�ö��ַ����� | |
| C�� | ʯ�͵��ѻ����ѽⶼ���������仯 | |
| D�� | ��Ȼ�������������ڿ������������Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3��NaOH | B�� | NaAl02��H2S04 | C�� | Na2C03��HCl | D�� | NaHS04��Ba��OH��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
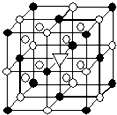 ����ʯ����ѧʽΪNa3AlF6���Ľṹ��Ԫ��ͼ��ʾ����֪����ʯ����ʱ���뷽��ʽΪNa3AlF6=3Na++AlF6-����λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ��������������ǣ�������
����ʯ����ѧʽΪNa3AlF6���Ľṹ��Ԫ��ͼ��ʾ����֪����ʯ����ʱ���뷽��ʽΪNa3AlF6=3Na++AlF6-����λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ��������������ǣ�������| A�� | Na+ | B�� | Al3+ | C�� | F- | D�� | AlF6- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | CO2 | D�� | ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.5L0.1mol/L��NaCl��Һ | B�� | 1L0.2mol/L��MgCl2��Һ | ||
| C�� | 1L0.3mol/L������Һ | D�� | 100mL0.2mol/L��AlCl3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{a+b-2c}{4}$kJ | B�� | $\frac{a+2b-4c}{8}$kJ | C�� | $\frac{b-a-2c}{4}$kJ | D�� | $\frac{2b-a-4c}{8}$kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | Ŀ�� | ���ӷ���ʽ | |
| A | �кͷ� | ��H2SO4�кͼ��Է�ˮ | H++OH-�TH2O |
| B | ������ | ��ȥˮ�������� | Al3++H2O�TAl��OH��3��+H+ |
| C | ������ | ��ȥ��ˮ�е�Hg2+ | Hg2++Na2S�THgS��+Na+ |
| D | ������ | ���� | Cl2+2OH-�TCl-+ClO-+H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com