

��֪CaC2��ˮ��Ӧ�Ļ�ѧ����ΪCaC2+2H2O![]() Ca(OH)2+HC��CH�����ش��������⣺
Ca(OH)2+HC��CH�����ش��������⣺
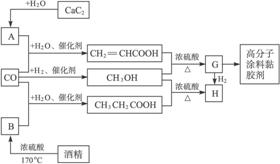
(1)д��G�Ľṹ��ʽ��_____________________
(2)д��ͼ������CH3OH(�״�)�Ļ�ѧ����ʽ��д����Ũ��������������H�Ļ�ѧ����ʽ��_______________________________________________________________
(3)ָ���������յ��ŵ���ʲô��__________________________________________
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| Ũ���ᡢ�� |
| ���� |
| ���� |
| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� |
| Ũ����� |
| ���� |
| ���� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CO�����Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϡ���ͼ����ijЩ���л����ڵ��¡���ѹ�ʹ��������ºϳɾ����������ܵ�װ���Ը߷���Ϳ�ϡ�𤽺���Ļ������̡�
��֪CaC2��ˮ��Ӧ�Ļ�ѧ����ΪCaC2+2H2O![]() Ca��OH��2+HC��CH�����ش��������⣺
Ca��OH��2+HC��CH�����ش��������⣺
��1��д��G�Ľṹ��ʽ��
��2��д��ͼ������CH3OH���״����Ļ�ѧ����ʽ��д����Ũ��������������H�Ļ�ѧ����ʽ��
��3��ָ���������յ��ŵ���ʲô��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�갲��ʡ������ѧ��������Ծ������������ۺϻ�ѧ ���ͣ������
��14�֣�CO�����Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϡ�����������������һ�ֵ��µ�ѹ�����գ���ijЩ���л��ᆳ���ʻ�����Ӧ�����������һ������������ܵ�װ���Ը߷���Ϳ�ϡ�ճ�ϼ��ȡ�����ͼ��ʾ��
 |

 ��4��д������ת���Ļ�ѧ����ʽ��
��4��д������ת���Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com