
 Ä³Ń§ÉśÓĆ0.1000mol•L-1±ź×¼NaOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬Ęä²Ł×÷æÉ·Ö½āĪŖČēĻĀ¼ø²½£ŗ
Ä³Ń§ÉśÓĆ0.1000mol•L-1±ź×¼NaOHČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖį£¬Ęä²Ł×÷æÉ·Ö½āĪŖČēĻĀ¼ø²½£ŗ| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż£Ø/mL£© | ±ź×¼NaOHČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°¶ĮŹż£Ø/mL£© | µĪ¶Øŗó¶ĮŹż£Ø/mL£© | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 0.00 | 22.99 |
| 3 | 25.00 | 0.20 | 20.19 |
·ÖĪö £Ø1£©øł¾ŻµĪ¶Ø¹ÜµÄĢŲµć·ÖĪö£»
£Ø2£©øł¾ŻÖŠŗĶµĪ¶ØÓŠ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢Č”“ż²āŅŗ²¢¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČ²Ł×÷£»
£Ø3£©ÓƱź×¼NaOHČÜŅŗČóĻ“µĪ¶Ø¹Ü£¬·ĄÖ¹²śÉśĪó²ī£»
£Ø4£©ČēČÜŅŗŃÕÉ«±ä»ÆĒŅ°ė·ÖÖÓÄŚ²»±äÉ«£¬æÉĖµĆ÷“ļµ½µĪ¶ØÖÕµć£»
£Ø5£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$ÅŠ¶Ļ²»µ±²Ł×÷¶ŌĻą¹ŲĪļĄķĮæµÄÓ°Ļģ£»
£Ø6£©ĻČÅŠ¶ĻµĪ¶ØŹż¾ŻµÄÓŠŠ§ŠŌ£¬Ēó³ö±ź×¼ŅŗµÄĘ½¾łĢå»ż£¬Č»ŗóøł¾Ż¹ŲĻµŹ½HCl”«NaOHĄ“¼ĘĖć³öŃĪĖįµÄÅØ¶Č£»
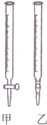
½ā“š ½ā£ŗ£Ø1£©ĖįŹ½µĪ¶Ø¹ÜĻĀ¶ĖŹĒ²£Į§»īČū£¬¼īŹ½µĪ¶Ø¹ÜĻĀ¶ĖŹĒĻšĘ¤£»
¹Ź“š°øĪŖ£ŗ¼×£»
£Ø2£©ÖŠŗĶµĪ¶Ø°“ÕÕ¼ģĀ©”¢Ļ“µÓ”¢ČóĻ“”¢×°Ņŗ”¢Č”“ż²āŅŗ²¢¼ÓÖøŹ¾¼Į”¢µĪ¶ØµČĖ³Šņ²Ł×÷£¬ŌņÕżČ·µÄĖ³ŠņĪŖ£ŗBDCEAF£»
¹Ź“š°øĪŖ£ŗBDCEAF£»
£Ø3£©ÓƱź×¼NaOHČÜŅŗČóĻ“µĪ¶Ø¹Ü2”«3“Ī£¬·ĄÖ¹µĪ¶Ø¹ÜÄŚ±Śø½×ŵÄĖ®½«±ź×¼ČÜŅŗĻ”ŹĶ¶ų“ųĄ“Īó²ī£»
¹Ź“š°øĪŖ£ŗ·ĄÖ¹µĪ¶Ø¹ÜÄŚ±Śø½×ŵÄĖ®½«±ź×¼ČÜŅŗĻ”ŹĶ¶ų“ųĄ“Īó²ī£»
£Ø4£©±¾ŹµŃéŹĒÓĆNaOHµĪ¶ØŃĪĖįČÜŅŗ£¬ÓĆ·ÓĢŖ×÷ÖøŹ¾¼Į£¬ĖłŅŌÖÕµćŹ±£¬ĻÖĻóŹĒµ±ČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«£¬ĒŅŌŚ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
¹Ź“š°øĪŖ£ŗČÜŅŗÓÉĪŽÉ«±äĪŖĒ³ŗģÉ«£¬ĒŅ°ė·ÖÖÓÄŚ²»ĶŹÉ«£»
£Ø5£©A”¢¼īŹ½µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“¾»ŗóĪ“ÓƱź×¼ŅŗČóĻ“£¬Ōņ±ź×¼ŅŗÅØ¶Č»į¼õŠ”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«øߣ¬¹ŹAÕżČ·£»
B”¢ŌŚÕńµ“׶ŠĪĘæŹ±²»É÷½«ĘæÄŚČÜŅŗ½¦³ö£¬“ż²āŅŗµÄĪļÖŹµÄĮæĘ«Š”£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹB“ķĪó£»
C”¢µĪ¶ØĒ°¼īŹ½µĪ¶Ø¹Ü¼ā¶ĖĘųÅŻĪ“Åųż£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬Ōģ³ÉV£Ø±ź×¼£©Ę«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«øߣ¬¹ŹCÕżČ·£»
D”¢µĪ¶ØĒ°¶ĮŹżÕżČ·£¬µĪ¶Øŗóø©ŹÓµĪ¶Ø¹Ü¶ĮŹż£¬Ōģ³ÉV£Ø±ź×¼£©Ę«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©”ĮV£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹD“ķĪó£»
¹ŹŃ”AC£»
£Ø6£©Čż“ĪµĪ¶ØĻūŗıź×¼ŅŗµÄĢå»ż·Ö±šĪŖ£ŗ20.01mL£¬22.99mL£¬19.99mL£¬µŚ¶ž“ĪµĪ¶ØŹż¾ŻĪó²ī¹ż“ó£¬Ó¦øĆÉįĘś£¬ĘäĖüĮ½“ĪĻūŗĵıź×¼ŅŗµÄĘ½¾łĢå»żĪŖ
20.00mL£¬
HCl”«NaOH
1 1
C£ØHCl£©”Į25.00mL 0.1000mol•L-1”Į20.00mL£»
C£ØHCl£©=$\frac{0.1000mol•{L}^{-1}”Į20mL}{25mL}$=0.0800mol•L-1£¬
¹Ź“š°øĪŖ£ŗ0.0800mol•L-1£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖĖį¼īÖŠŗĶµĪ¶ØµÄ²Ł×÷²½Öč”¢µĪ¶Ø¹ÜµÄŹ¹ÓĆ”¢Īó²ī·ÖĪö£¬ÄѶČÖŠµČ£¬ÕĘĪÕÖŠŗĶµĪ¶ØµÄŌĄķŹĒ½āĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2SO4 | B£® | KOH | C£® | FeCl3 | D£® | Ba £ØNO3£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH4N03ŹĒĄė×Ó»ÆŗĻĪļ£¬KN03ŹĒ¹²¼Ū»ÆŗĻĪļ | |
| B£® | NH4N03ŹĒ¹²¼Ū»ÆŗĻĪļ£¬KN03ŹĒĄė×Ó»ÆŗĻĪļ | |
| C£® | NH4N03ŗĶKN03¶¼ŹĒĄė×Ó»ÆŗĻĪļ | |
| D£® | NH4N03ŗĶKN03¶¼ŹĒ¹²¼Ū»ÆŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
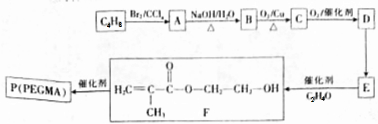
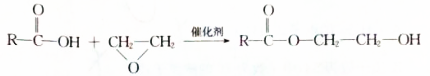
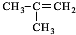 £®
£®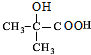 $”ś_{”÷}^{ÅØĮņĖį}$
$”ś_{”÷}^{ÅØĮņĖį}$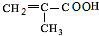 +H2O£¬øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶŹĒĻūČ„·“Ó¦£®
+H2O£¬øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶŹĒĻūČ„·“Ó¦£®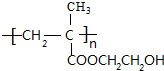 £®
£®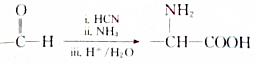 £®Éč¼ĘÓÉBŗĻ³É
£®Éč¼ĘÓÉBŗĻ³É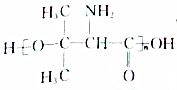 µÄĀ·Ļߣ®ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ēė²Īæ¼ČēĻĀŠĪŹ½£®
µÄĀ·Ļߣ®ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ēė²Īæ¼ČēĻĀŠĪŹ½£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼Ó½šŹōÄĘ£¬ÓŠĘųĢå²śÉśµÄŹĒŅŅ“¼ | |
| B£® | ¼ÓĖ®£¬»„ČܵďĒŅŅ“¼ | |
| C£® | µćČ¼£¬ČŻŅ×Č¼ÉÕµÄŹĒŅŅ“¼ | |
| D£® | ŗĶÅØĮņĖį»ģŗĻ¹²ČČÖĮ170”ę£¬ÓŠŅŅĻ©²śÉśµÄŹĒŅŅ“¼ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
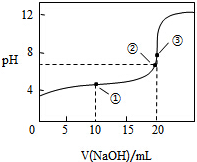 ³£ĪĀĻĀ£¬ÓĆ0.1000mol•L-1 NaOHČÜŅŗµĪ¶Ø20.00mL0.1000mol•L-1 CH3COOHČÜŅŗĖłµĆµĪ¶ØĒśĻßČēĶ¼£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
³£ĪĀĻĀ£¬ÓĆ0.1000mol•L-1 NaOHČÜŅŗµĪ¶Ø20.00mL0.1000mol•L-1 CH3COOHČÜŅŗĖłµĆµĪ¶ØĒśĻßČēĶ¼£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | µć¢ŪČÜŅŗĻŌ¼īŠŌµÄŌŅņŹĒ CH3COO-+H2OØTCH3COOH+OH- | |
| B£® | µć¢ŚŹ±ČÜŅŗÖŠc£ØNa+£©“óÓŚc£ØCH3COO-£© | |
| C£® | µć¢ŁČÜŅŗÖŠ c£ØCH3COOH£©+c£ØH+£©£¾c£ØCH3COO-£©+c£ØOH-£© | |
| D£® | ŌŚÖšµĪ¼ÓČėNaOHČÜŅŗÖĮ40mLµÄ¹ż³ĢÖŠ£¬Ė®µÄµēĄė³Ģ¶ČĻČŌö“óŗó¼õŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | pH=3µÄ¶žŌŖČõĖįH2RČÜŅŗÓėp=11µÄNaOHČÜŅŗ»ģŗĻŗ󣬻ģŗĻŅŗµÄpHµČÓŚ7£¬Ōņ·“Ó¦ŗóµÄ»ģŗĻŅŗÖŠ£ŗ2c£ØR2-£©+c£ØHR-£©=£ØNa+£© | |
| B£® | Čō0.3mol•L-1HYČÜŅŗÓė0.3mol•L-1NaOHČÜŅŗµČĢå»ż»ģŗĻŗó£¬ČÜŅŗµÄpH=9£¬Ōņ£ŗc£ØOH-£©-c£ØHY£©=£ØH+£©=1”Į10-9mol•L-1 | |
| C£® | 0.2mol•L-1HClČÜŅŗÓėµČĢå»ż0.05mol•L-1Ba£ØOH£©2ČÜŅŗ»ģŗĻŗó£¬ČÜŅŗµÄpH=1 | |
| D£® | 0.1mol•L-1Na2SÓė0.1mol•L-1NaHSµČĢå»ż»ģŗĻ£ŗ3c£ØNa+£©-2c£ØHS-£©=2c£ØS2-£©+2c£ØH2S£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
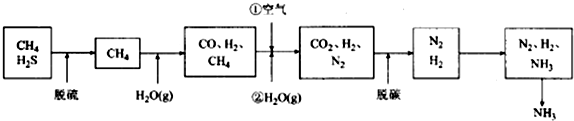
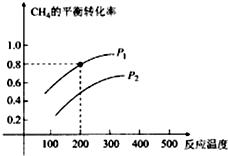
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com