
��12�֣�����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֡�K2Cr2O7��CrO3�����������ӡȾ�����ϡ���Ƶȹ�ҵ�У��ǹ�ҵ����ɸ���Ⱦ����Ҫԭ���ڱ���ġ������ҡ��¼��У�������Ϊ�ù�ҵƤ����½��ϻ���ƤЬ��Ϊԭ���ƳɵĹ�ҵ������ð���ʳ�������Ƴɽ��ң���ɽ����ڵĸ����س��ꡣ
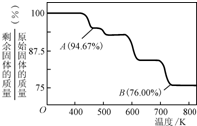
��1��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯��ͼ��ʾ��

��A ��ʱʣ�����ijɷ��� ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ ��
��2��Cr(��)��Ҫ��CrO42����Cr2O72����̬���ڣ������������¾��к�ǿ�������ԣ���������Һ�д�������ת����CrO42��(��ɫ)+2H+  Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ��
��
Cr2O72��(��ɫ��+H2O��K=4.2��1014����Ҫʹ��Һ�ɻ�ɫ���ɫ����Ӧ��ȡ�Ĵ�ʩ��
��
A����NaOH B�������� C�������� D����AgNO3
��3����ҵ��ˮ�г�����һ������Cr(��)�����Խϴ����ǻ�����༰��̬ϵͳ�����ܴ���������������֮һ�ǽ���Cr2O72���ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2����Cr2O72��������Ӧ�����ɵ�Fe3����Cr3������������OH���������Fe(OH)3��Cr(OH)3�����Գ�ȥ[��֪KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31]��
�ٵ������� NaCl �������� ��
��д�������ĵ缫��Ӧʽ ��
��д��Fe2����Cr2O72��������Ӧ����Fe3����Cr3�������ӷ�Ӧ����ʽ ��
����֪�������Һ��c(Fe3��)=2.0��10��13 mol��L��1������Һ��c(Cr3��)Ϊ mol��L��1��
��1����Cr3O8 ����CrO2.67) ��2�֣� �� 4CrO32Cr2O3��3O2����2�֣�
��2��C ��2�֣� (3) �� ��ǿ��Һ�ĵ����� ��1�֣� ��Fe-2e��=Fe2+ ��1�֣�
��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O ��2�֣� ��3.0��10��6 ��2�֣�
����������1���ټ��������100g�����ʵ�����1mol��������ԭ����3mol��A�������94.67g�����ٵ�������5.33g�������ٵ���ԭ����0.333mol�����Բ���Cr����ԭ�ӵ����ʵ���֮����1�U��3��1/3��=3�U8,���Ի�ѧʽΪCr3O8��
�ڼ��ȵ� 750K ʱ��ʣ�����������76.0g�����ݢټ���ԭ��ͬ�����Եó���ʱ������Cr2O3��ʣ���ʽΪ4CrO32Cr2O3��3O2����
��2��Ҫʹ��Һ�ɻ�ɫ���ɫ����ƽ��Ӧ��������Ӧ�����ƶ�����������������Ũ�ȿ��ԣ�A����ȷ������Ũ�����ܱ�����������ѡ��B��C��ȷ��D�л����ɸ��������������淴Ӧ�����ƶ�������ȷ����ѡC��
��3��������ˮ�ĵ��������ϲ���Ȼ�����ǿ����ʣ�����ǿ��Һ�ĵ����ԡ�
������ʧȥ���ӣ��������������Է���ʽΪFe-2e��=Fe2+ ��
�۸��ݷ�Ӧ����������֪������ʽΪ6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O��
�ܸ��ݶ��ߵ��ܶȻ���������ʽ��֪����Һ��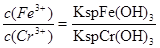 �����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ
�����ڵ������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ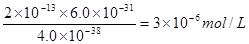 ��
��


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
�����������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�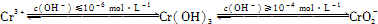
2- 7 |
2- 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У�
����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ��������⣨������ѧ�Ծ��������棩 ���ͣ������
��11�֣�����ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
��1��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬCrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ��
____________ ��
��2��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��
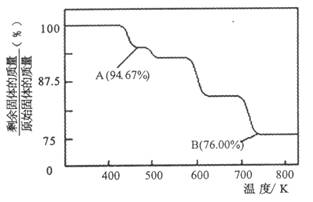
��A ��ʱʣ�����ijɷ���_________ ���ѧʽ����
�ڴӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ______________________ _��
��3��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10��38��KspCr(OH)3��6.0��10��31]��
�ٵ������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L��1������Һ��c(Cr3+)Ϊ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ��ͳ����ѧ�Ծ� ���ͣ������
(16��)������ѧ�ḻ��ʣ����ڸ�����Ⱥã����������������������棬ͬ��������ɸ������ܵIJ���֣�CrO3���������ڵ�ƹ�ҵ�С�
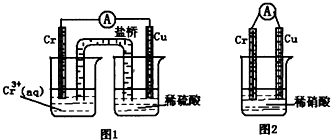
��1������ͼװ���У��۲쵽ͼ1װ��ͭ�缫�ϲ�����������ɫ���ݣ���ͼ 2װ����ͭ�缫����������������缫�ϲ���������ɫ���塣��ͼ 1 ֪�������Ļ�Ա�ͭ_____(��ǿ����)��ͼ 2װ���и��缫�ĵ缫��Ӧʽ

��2��CrO3����ǿ�����ԣ������л����ƾ���ʱ�����ҷ�Ӧ�����Ż����ù������Ҵ������������ᣬ CrO3����ԭ����ɫ�������[Cr2(SO4)3]����÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________________��
(3)����ƽ�⣺2CrO42������ɫ��+2H+ Cr2O72������ɫ��+H2O
Cr2O72������ɫ��+H2O
����ƽ����ϵ��pH=2������Һ�� ɫ.
����˵���ڢٲ���Ӧ��ƽ��״̬���� ��
a��Cr2O72����CrO42����Ũ����ͬ b��2v (Cr2O72��) =v (CrO42��) c����Һ����ɫ����
��4��CrO3�� K2Cr2O7��������ˮ�����ǹ�ҵ����ɸ���Ⱦ����Ҫԭ������������֮һ�ǽ�����6�� Cr �ķ�ˮ��������ڣ�����������������������NaCl���е�⣺���������ɵ�Fe2+��Cr2O72��������Ӧ�����ɵ�Fe3+��Cr3+����������OH��������� Fe(OH)3 ��Cr(OH)3������ȥ[��֪ KspFe(OH)3��4.0��10-38��KspCr(OH)3��6.0��10-31]��
�ٵ������� NaCl ��������__________________________��
����֪�������Һ��c(Fe3+)Ϊ2.0��10��13 mol��L1������Һ��c(Cr3+)Ϊ____ mol��L-1��
��5��CrO3�����ȶ��Խϲ����ʱ�ֽ⣬�������������¶ȵı仯����ͼ��ʾ��

�ӿ�ʼ���ȵ� 750K ʱ�ܷ�Ӧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com