��16�֣�Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
��ʵ���ã�5g�״��������г��ȼ�����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��____________________________________��
����ͼ��ij�ʼDZ������ü״�ȼ�ϵ�صĽṹʾ��ͼ��
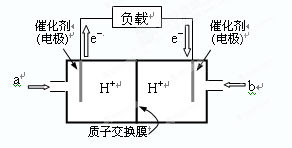
�ŵ�ʱ�״�Ӧ��______��ͨ�루�a����b����������ڲ�H����_____������ҡ����ƶ���д����ظ����ĵ缫��Ӧʽ��_______________________________��
������̬��̬ԭ���γ�1mol��ѧ���ͷŵ���������м��ܡ��ӻ�ѧ���ĽǶȷ�������ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ�����У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��ѧ��
| H��H
| N��H
| N��N
|
����/kJ��mol��1
| 436
| a
| 945
|
��֪��ӦN
2(g)��3H
2(g)

2NH
3(g) ��
H����93 kJ��mol
��1���Ը��ݱ������м������ݼ���
a����ֵ��_______________��
�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C(s��ʯī)��O
2(g)��CO
2(g) ��
H1��
��393.5kJ��mol
��12H
2(g)��O
2(g)��2H
2O(l) ��
H2����571.6kJ��mol
��12C
2H
2(g)��5O
2(g)��4CO
2(g)��2H
2O(l) ��
H3��
��2599kJ��mol
��1���ݸ�˹���ɣ�����2C(s��ʯī)��H
2(g)��C
2H
2(g)��Ӧ���ʱ��
H��________��


 2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݼ���a����ֵ��_______________��
2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݼ���a����ֵ��_______________��