
°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā
(1)ŅŃÖŖŌŚ³£ĪĀ³£Ń¹ĻĀ£ŗ
¢Ł2CH3OH(l)£«3O2(g)===2CO2(g)£«4H2O(g)
¦¤H£½£1 275.6 kJ·mol£1
¢ŚH2O(l)===H2O(g)””¦¤H£½£«44.0 kJ·mol£1
Š“³ö±ķŹ¾¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½_______________________________________”£
(2)ŅŃÖŖ£ŗCH3OH(g)£« O2(g)??CO2(g)£«2H2(g)
O2(g)??CO2(g)£«2H2(g)
¦¤H1£½£192.9 kJ·mol£1
H2(g)£« O2(g)??H2O(g)
O2(g)??H2O(g)
¦¤H2£½£120.9 kJ·mol£1
Ōņ¼×“¼ÓėĖ®ÕōĘų“ß»ÆÖŲÕū·“Ó¦µÄģŹ±ä¦¤H3£½__________________”£
(3)±½ŅŅĻ©ŹĒÖŲŅŖµÄ»ł“”ÓŠ»śŌĮĻ”£¹¤ŅµÖŠÓĆŅŅ±½(C6H5—CH2CH3)ĪŖŌĮĻ£¬²ÉÓĆ“ß»ÆĶŃĒāµÄ·½·ØÖĘČ”±½ŅŅĻ©(C6H5—CH===CH2)µÄ·“Ó¦·½³ĢŹ½ĪŖ
C6H5—CH2CH3(g)??C6H5—CH===CH2(g)£«H2(g)””¦¤H1
ŅŃÖŖ£ŗ3C2H2(g)??C6H6(g)””¦¤H2
C6H6(g)£«C2H4(g)??C6H5—CH2CH3(g)””¦¤H3
Ōņ·“Ó¦3C2H2(g)£«C2H4(g)??C6H5—CH===CH2(g)£«H2(g)µÄ¦¤H£½____________”£
(4)°±µÄŗĻ³ÉŹĒ×īÖŲŅŖµÄ»Æ¹¤Éś²śÖ®Ņ»”£
¹¤ŅµÉĻŗĻ³É°±ÓƵÄH2ÓŠ¶ąÖÖÖĘČ”µÄ·½·Ø£ŗ
¢ŁÓĆ½¹ĢæøśĖ®·“Ó¦£ŗC(s)£«H2O(g)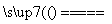 CO(g)£«H2(g)£»
CO(g)£«H2(g)£»
¢ŚÓĆĢģČ»ĘųøśĖ®ÕōĘų·“Ó¦£ŗCH4(g)£«H2O(g) CO(g)£«3H2(g)
CO(g)£«3H2(g)
ŅŃÖŖÓŠ¹Ų·“Ó¦µÄÄÜĮæ±ä»ÆČēĻĀĶ¼ĖłŹ¾£¬Ōņ·½·Ø¢ŚÖŠ·“Ó¦µÄ¦¤H£½____________”£
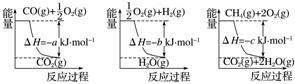
(5)¼×“¼ŹĒŅ»ÖÖÓĆĶ¾¹ć·ŗµÄ»Æ¹¤ŌĮĻ”£
¹¤ŅµÉĻ³£ÓĆĻĀĮŠĮ½ÖÖ·“Ó¦Öʱø¼×“¼£ŗ
¢ŁCO(g)£«2H2(g)??CH3OH(g)
¦¤H1£½£90.1 kJ·mol£1
¢ŚCO2(g)£«3H2(g)??CH3OH(g)£«H2O(l)””¦¤H2
ŅŃÖŖ£ŗCO(g)£«H2O(g)===CO2(g)£«H2(g)
¦¤H3£½£41.1 kJ·mol£1
H2O(l)===H2O(g)””¦¤H4£½£«44.0 kJ·mol£1
Ōņ¦¤H2£½__________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µē½āNOÖʱøNH4NO3£¬Ę乤×÷ŌĄķČēĶ¼ĖłŹ¾£¬ĪŖŹ¹µē½ā²śĪļČ«²æ×Ŗ»ÆĪŖNH4NO3£¬Šč²¹³äĪļÖŹA£¬AŹĒ________£¬ĖµĆ÷ĄķÓÉ£ŗ______________________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiHæÉ×÷·É“¬µÄČ¼ĮĻ£¬ŅŃÖŖĻĀĮŠ·“Ó¦£ŗ
¢Ł2Li(s)£«H2(g)===2LiH(s)””¦¤H£½£182 kJ·mol£1
¢Ś2H2(g)£«O2(g)===2H2O(l)””¦¤H£½£572 kJ·mol£1
¢Ū4Li(s)£«O2(g)===2Li2O(s)””¦¤H£½£1 196 kJ·mol£1
ŹŌŠ“³öLiHŌŚO2ÖŠČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖ£ŗ25 ”ę”¢101 kPaŹ±£¬Mn(s)£«O2(g)===MnO2(s)””¦¤H£½£520 kJ·mol£1
S(s)£«O2(g)===SO2(g)””¦¤H£½£297 kJ·mol””Mn(s)£«S(s)£«2O2(g)===MnSO4(s)
¦¤H£½£1 065 kJ·mol£1
SO2ÓėMnO2·“Ӧɜ³ÉĪŽĖ®MnSO4µÄČČ»Æѧ·½³ĢŹ½ŹĒ__________________________”£
(2)[2014·Õć½Ąķ×Ū£¬27(3)]ĆŗĢæČ¼ÉÕ¹ż³ĢÖŠ»įŹĶ·Å³ö“óĮæµÄSO2£¬ŃĻÖŲĘĘ»µÉśĢ¬»·¾³”£²ÉÓĆŅ»¶ØµÄĶŃĮņ¼¼ŹõæÉŅŌ°ŃĮņŌŖĖŲŅŌCaSO4µÄŠĪŹ½¹Ģ¶Ø£¬“Ó¶ų½µµĶSO2µÄÅÅ·Å”£µ«ŹĒĆŗĢæČ¼ÉÕ¹ż³ĢÖŠ²śÉśµÄCOÓÖ»įÓėCaSO4·¢Éś»Æѧ·“Ó¦£¬½µµĶĮĖĶŃĮņŠ§ĀŹ”£Ļą¹Ų·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
CaSO4(s)£«CO(g)??CaO(s)£«SO2(g)£«CO2(g)
¦¤H1£½218.4 kJ·mol£1(·“Ó¦¢ń)
CaSO4(s)£«4CO(g)??CaS(s)£«4CO2(g)
¦¤H2£½£175.6 kJ·mol£1(·“Ó¦¢ņ)
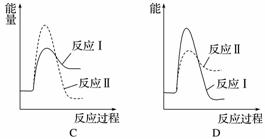
¼ŁÉčijĪĀ¶ČĻĀ£¬·“Ó¦¢ńµÄĖŁĀŹ(v1)“óÓŚ·“Ó¦¢ņµÄĖŁĀŹ(v2)£¬ŌņĻĀĮŠ·“Ó¦¹ż³ĢÄÜĮæ±ä»ÆŹ¾ŅāĶ¼ÕżČ·µÄŹĒ______”£
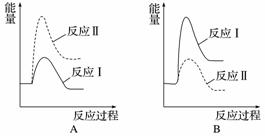
(3)[2014·¹ć¶«Ąķ×Ū£¬31(1)]ÓĆCaSO4“śĢęO2ÓėČ¼ĮĻCO·“Ó¦£¬¼ČæÉĢįøßČ¼ĮĻŠ§ĀŹ£¬ÓÖÄܵƵ½øß“æCO2£¬ŹĒŅ»ÖÖøߊ§”¢Ēå½ą”¢¾¼ĆµÄŠĀŠĶČ¼ÉÕ¼¼Źõ£¬·“Ó¦¢ŁĪŖÖ÷·“Ó¦£¬·“Ó¦¢ŚŗĶ¢ŪĪŖø±·“Ó¦”£
¢Ł1/4CaSO4(s)£«CO(g)??1/4CaS(s)£«CO2(g)
¦¤H1£½£47.3 kJ·mol£1
¢ŚCaSO4(s)£«CO(g)??CaO(s)£«CO2(g)£«SO2(g)
¦¤H2£½£«210.5 kJ·mol£1
¢ŪCO(g)??1/2C(s)£«1/2CO2(g)
¦¤H3£½£86.2 kJ·mol£1
·“Ó¦2CaSO4(s)£«7CO(g)??CaS(s)£«CaO(s)£«6CO2(g)£«C(s)£«SO2(g)µÄ¦¤H£½__________(ÓƦ¤H1”¢¦¤H2ŗĶ¦¤H3±ķŹ¾)”£
(4)[2014·“óøŁČ«¹ś¾ķ£¬28(1)]»ÆŗĻĪļAX3ŗĶµ„ÖŹX2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÉś³É»ÆŗĻĪļAX5”£»Ų“šĻĀĮŠĪŹĢā£ŗ
ŅŃÖŖAX3µÄČŪµćŗĶ·Šµć·Ö±šĪŖ£93.6 ”ęŗĶ76 ”ę£¬AX5µÄČŪµćĪŖ167 ”ę”£ŹŅĪĀŹ±AX3ÓėĘųĢåX2·“Ӧɜ³É1 mol AX5£¬·Å³öČČĮæ123.8 kJ”£øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________________”£
(5)[2014·Ģģ½ņĄķ×Ū£¬7(4)]¾§Ģå¹č(ČŪµć1 410 ”ę)ŹĒĮ¼ŗƵİėµ¼Ģå²ÄĮĻ”£ÓÉ“Ö¹čÖĘ“æ¹č¹ż³ĢČēĻĀ£ŗ
Š“³öSiCl4µÄµē×ÓŹ½£ŗ______£»ŌŚÉĻŹöÓÉSiCl4ÖĘ“æ¹čµÄ·“Ó¦ÖŠ£¬²āµĆĆæÉś³É1.12 kg“æ¹čŠčĪüŹÕa kJČČĮ棬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ______________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ25 ”ę”¢101 kPaĻĀ£¬1 g¼×“¼Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·ÅČČ22.68 kJ£¬ĻĀĮŠČČ»Æѧ·½³ĢŹ½ÕżČ·µÄŹĒ(””””)
A£®CH3OH(l)£« O2(g)===CO2(g)£«2H2O(l)””¦¤H£½£«725.76 kJ·mol£1
O2(g)===CO2(g)£«2H2O(l)””¦¤H£½£«725.76 kJ·mol£1
B£®2CH3OH(l)£«3O2(g)===2CO2(g)£«4H2O(l)””¦¤H£½£1 451.52 kJ·mol£1
C£®2CH3OH(l)£«3O2(g)===2CO2(g)£«4H2O(l)””¦¤H£½£725.76 kJ·mol£1
D£®2CH3OH(l)£«3O2(g)===2CO2(g)£«4H2O(l)””¦¤H£½£«1 451.52 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĪĀ¶ČĻĀ£¬Ė®“ęŌŚH2OH£«£«OH£””¦¤H>0µÄĘ½ŗā£¬ĻĀĮŠŠšŹöŅ»¶ØÕżČ·µÄŹĒ(””””)
A£®ĻņĖ®ÖŠµĪČėÉŁĮæĻ”ŃĪĖį£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬Kw¼õŠ”
B£®½«Ė®¼ÓČČ£¬KwŌö“ó£¬pH¼õŠ”
C£®ĻņĖ®ÖŠ¼ÓČėÉŁĮæ¹ĢĢåCH3COONa£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬c(H£«)½µµĶ
D£®ĻņĖ®ÖŠ¼ÓČėÉŁĮæ¹ĢĢåĮņĖįÄĘ£¬c(H£«)£½10£7 mol·L£1£¬Kw²»±ä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)Ģå»żĻąĶ¬£¬ÅØ¶Č¾łĪŖ0.2 mol·L£1µÄŃĪĖįŗĶCH3COOHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶ10±¶£¬ČÜŅŗµÄpH·Ö±š±ä³ÉmŗĶn£¬ŌņmÓėnµÄ¹ŲĻµĪŖ________”£
(2)Ģå»żĻąĶ¬£¬ÅØ¶Č¾łĪŖ0.2 mol·L£1µÄŃĪĖįŗĶCH3COOHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶm±¶”¢n±¶£¬ČÜŅŗµÄpH¶¼±ä³É3£¬ŌņmÓėnµÄ¹ŲĻµĪŖ________________”£
(3)Ģå»żĻąĶ¬£¬pH¾łµČÓŚ1µÄŃĪĖįŗĶCH3COOHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶm±¶”¢n±¶£¬ČÜŅŗµÄpH¶¼±ä³É3£¬ŌņmÓėnµÄ¹ŲĻµĪŖ________________”£
(4)Ģå»żĻąĶ¬£¬pH¾łµČÓŚ13µÄ°±Ė®ŗĶNaOHČÜŅŗ£¬·Ö±š¼ÓĖ®Ļ”ŹĶm±¶”¢n±¶£¬ČÜŅŗµÄpH¶¼±ä³É9£¬ŌņmÓėnµÄ¹ŲĻµĪŖ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆI2µÄŃõ»ÆŠŌæɲā¶ØøÖĢśÖŠĮņµÄŗ¬Į攣×ö·ØŹĒ½«øÖŃłÖŠµÄĮņ×Ŗ»Æ³ÉH2SO3£¬Č»ŗóÓĆŅ»¶ØÅØ¶ČµÄI2ČÜŅŗ½ųŠŠµĪ¶Ø£¬ĖłÓĆÖøŹ¾¼ĮĪŖ________£¬µĪ¶Ø·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com