
ij������A������ʽΪC8H10 ��ij����������X������ʽΪC15H14O3����ʹFeCl3��Һ����ɫ��J��������������Ϊ��λ��ȡ��������һ�������������µ�ת����ϵ (������ȥ)��
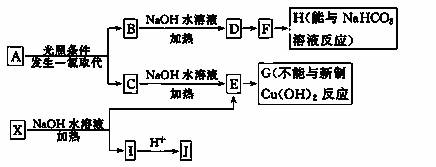
(1)J�������ĺ��������ŵ�����Ϊ________________________________________��
(2)E��H��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��Ӧ������______________��
(3)B��C�Ļ������NaOH�Ҵ���Һ�м��ȿ�������ͬһ���л���M����MΪ����ϳɵĸ߷��ӻ������������____________��
(4)��֪J�ж���ͬ���칹�壬д��һ�ַ����������ʵ�J��ͬ���칹��Ľṹ��ʽ������FeCl3��Һ��������ɫ����������Cu(OH)2����Һ���ò�����ɫ�������۱����ϵ�һ±������2�֣�
�ܺ˴Ź���������ʾ�����ֲ��壬�Ҳ�������֮��Ϊ1��2��2��1��________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ú����ˮ����Na2S2O3��5H2O��ʵ���ҿ�������װ��(��ȥ���ּг�����)ģ���������̡�
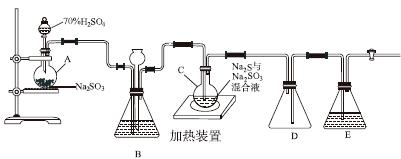
��ƿC�з�����Ӧ���£�
Na2S(aq)��H2O(l)��SO2(g)===Na2SO3(aq)��H2S(aq)��(��)
2H2S(aq)��SO2(g)===3S(s)��2H2O(l)��(��)
S(s)��Na2SO3(aq) Na2S2O3(aq)��(��)
Na2S2O3(aq)��(��)
(1)������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һ������________________��������װ�����������á�װ��D��������__________��װ��E��Ϊ________��Һ��
(2)Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ________��
(3)װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ��________��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ�����__________________________����֪��Ӧ(��)��Խ���������ƿC�з�Ӧ�ﵽ�յ��������__________________����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ��������������________��
a���ձ� b��������
c���Թ� d����ƿ
(4)��Ӧ��ֹ����ƿC�е���Һ������Ũ������ȴ�ᾧ��������Na2S2O3��5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�________________________________________��
��֪Na2S2O3��5H2O�����ֽ⣺S2O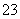 ��2H��===S����SO2����H2O
��2H��===S����SO2����H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���Na202����������ȷ���� ( )
A��Na202�����е��������������ӵ����ʵ���֮��Ϊ1��1
B��Na202����Ϊ���Ӿ��壬�Һ����Թ��ۼ�
C��Na202Ӧ�������ܷ⡢������������Ĺ��ƿ��
D��Na202�����ᷴӦ���������κ�ˮ���ɣ�ͬʱ��ų���ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȥ������������������(�����е�����)��ѡ�õ��Լ���װ�þ���ȷ����(����)
��.�Լ�����KMnO4/H������NaOH��Һ���۱���Na2CO3��Һ����H2O����Na����Br2/H2O����Br2/CCl4
��װ�ã�
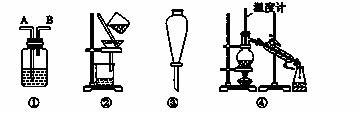
| ѡ�� | ���� | �Լ� | װ�� |
| A | C2H6(C2H4) | �� | �� |
| B | ��(����) | �� | �� |
| C | CH3COOC2H5(CH3COOH) | �� | �� |
| D | �ױ�(���ױ�) | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ñ�ȩ(CH3-CH2-CHO)��ȡ�۱�ϩ�Ĺ����з����ķ�Ӧ����Ϊ�� ��
��ȡ�� ����ȥ �ۼӾ� ������ ������ ��ԭ
A���ޢڢ� B���ݢڢ� C���٢ܢ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ij�л�����ӵı���ģ�ͣ��йظ����ʵ��ƶϲ���ȷ���ǣ� ��

A�������п��ܺ����ǻ� B�������п��ܺ����Ȼ�
C�������п��ܺ��а��� D�������ʵķ���ʽ����ΪC3H6O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���в����������۶�Ӧ��ϵ��ȷ��һ���ǣ� ��
| ѡ�� | ���� | ���� | ���� |
| A | �������м��뼸��ˮ������ȣ��ټ���Ũ���ᣬѸ�ٽ��� | ������ڣ�������ͣ��γ����ɶ������ | ֻ������Ũ�������ˮ�� |
| B | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
| C | �����������������Һ��ַ�Ӧ��������ϡ�����ữ���ټ�����������Һ | ���ɵ���ɫ���� | �������к�����Ԫ�� |
| D | ����ˮ���뱽�в������ | ��ˮ��ɫ | �����巢����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���еݱ��������ȷ����(����)
A��Na��Mg��Al�����������������࣬������ӵ�������������ǿ
B��P��S��Cl��������������ߣ���Ӧ����̬�⻯���ȶ�����ǿ
C��C��N��Oԭ�ӵİ뾶��������
D��Na��K��Rb�������ˮ�������������ǿ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com