
�����й�ʵ�����������ͽ��ͻ���۶���ȷ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ����ǵı�����Һ�м��뱥��Na2CO3��Һ | ��Һ����� | ���ԣ�����>̼�� |
| B | AgCl�����е���Na2S��Һ | ��ɫ�������ɫ | Ag2S��AgCl������ |
| C | ��AlCl3��Һ�е�������NaOH��Һ | ������ɫ���� | Al(OH)3�����ڼ� |
| D | �ò�����պȡij��Һ�ھƾ���������� | ����ʻ�ɫ | ˵������Һ��һ������Ԫ�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a(V2-V1) |
| V |
| a(V2-V1) |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.�����й�ʵ������У���������_____������ţ���
A.��������ƽ��ȡ11.70 gʳ��
B.����Ͳ��ȡ12.36 mL����
C.����ʽ�ζ�����ȡ21.20 mL 0.10 mol/L H2SO4��Һ
D.��200 mL����ƿ����500mL 0.1 mol/L NaCl��Һ
E.�ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ������������ˮ��ʪ����pH��ֽ�ϣ��������ɫ���Ƚ�
��.�������ʵ���Ũ��Ϊa mol/L�ı�����װ�ڵζ�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�����д���пհף�
��1����ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ�����______ ____��
��2�����ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ⶨ��NaOH���ʵ���Ũ�ȣߣߣ�ѡ�ƫ����ƫС������Ӱ�족��
������п��ʯī���������ϡ�������������ͭ��ɵĻ����Һ���������Һ���γɵ�ԭ����У������Ϸ����ķ�Ӧ�ĵ缫��Ӧʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���������ʮ���и߶���ѧ�����п��Ի�ѧ�� ���ͣ������
��.�����й�ʵ������У���������_____������ţ���
| A����������ƽ��ȡ11.70 gʳ�� |
| B������Ͳ��ȡ12.36 mL���� |
| C������ʽ�ζ�����ȡ21.20 mL 0.10 mol/L H2SO4��Һ |
| D����200 mL����ƿ����500 mL 0.1 mol/L NaCl��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ɹŰ������и߶����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣��������й�ʵ������У���������______________(�����) ��
A.��������ƽ��ȡ11.70gʳ�� B.����Ͳ��ȡ12.36mL����
C.����ʽ�ζ�����ȡ21.20mL0.10mol/L H2SO4��Һ
D.��200mL����ƿ����500mL0.1mol/L NaCl��Һ
E.�ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ������������ˮ��ʪ����pH��ֽ�ϣ��������ɫ���Ƚ�
���������ʵ���Ũ��Ϊa mol/L�ı�����ȥ�ⶨV mL NaOH��Һ�����ʵ���Ũ�ȣ�����д���пհף���1����ʽ�ζ���������ˮϴ����Ӧ�ý��еIJ�����_______________________��
��2����ͼ����ʽ�ζ�����Һ���ڵζ�ǰ��Ķ�����c (NaOH) = _____mol/L��

��3�����ڵζ�ǰ�ζ��ܼ��첿���������ݣ��ζ���ζ��ܼ��첿��������ʧ����ⶨ��NaOH���ʵ���Ũ��______����ѡ��ƫ��ƫС�������䡱��
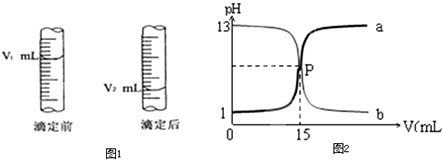
III����ͼ���������������Ƶĵζ�����a��b���������������գ�

����a���� ��Һ�ζ� ��Һ������b���� ��Һ�ζ� ��Һ��P�������Ϊ( )����������ʵ���Ũ��Ϊ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ĵ����һ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��.�����й�ʵ�����������˵������ȷ���� ������ĸ��
A���ζ�ʱ���۾�Ӧʼ��ע�ӵζ�����Һ��ı仯
B���ü�ʽ�ζ�����ȡ0.10 mol��L��1��KMnO4��Һ15.10 mL
C������к͵ζ�֮ǰ����ƿ������ˮϴ�����ɣ������ô���Һ��ϴ
D����pH��ֽ����ij��Һ��pHʱҪ�Ƚ���ֽ��ʪ
E���ζ��ܾ�����ˮϴ����ֱ��ע���Һ����ʹ��õĴ���ҺŨ��ƫ��
F���ù㷺pH��ֽ����H2SO4��Һ��pHʱ�����pH=3.2
G���ⶨ���ζ����ߣ���ʼʱ���Ժͼ�¼�ļ������СЩ���ζ����յ㸽����Ҫ��Щ
��.��1����������������Ӧ����1 molˮ��������241.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ ����֪H2O(l) �� H2O(g) ��H ����44 kJ��mol��1 ���״����33.6 L H2 ����Һ̬ˮʱ�ų��������� kJ��
��2����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̡���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4��P4O6�ķ��ӽṹ����ͼ��ʾ��
���ṩ���»�ѧ���ļ��ܣ�P-P 198 kJ��mol��1 P-O 360 kJ��mol��1,������������ԭ�Ӽ�ģ�O=O������Ϊ498 kJ��mol��1����P4+3O2 = P4O6�ķ�Ӧ�Ȧ�HΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com