
 ΩΣ–ΡΩλά÷ΦΌΤΎΉς“Β νΦΌΉς“ΒΈςΑ≤≥ωΑφ…γœΒΝ–¥πΑΗ
ΩΣ–ΡΩλά÷ΦΌΤΎΉς“Β νΦΌΉς“ΒΈςΑ≤≥ωΑφ…γœΒΝ–¥πΑΗ ΟϊΧβ―ΒΝΖœΒΝ–¥πΑΗ
ΟϊΧβ―ΒΝΖœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| ¥ΏΜ·ΦΝ |
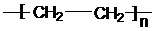
| ¥ΏΜ·ΦΝ |
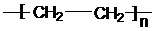
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
| ±Φδ | ΩΣ Φ | 8hΚσ | 16hΚσ | 24hΚσ | 32hΚσ | 40hΚσ | 48hΚσ |
| pH | 5.0 | 4.8 | 4.5 | 4.3 | 4.2 | 4.0 | 4.0 |
| ||
| ||
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2012-2013―ßΡξΗ Υύ ΓΗ Ι»“Μ÷–ΗΏΕΰœ¬―ßΤΎΒΎ“Μ¥Έ‘¬ΩΦΜ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚΦΤΥψΧβ
”–AΓΔBΝΫ÷÷ΧΰΘ§ΥϋΟ«ΒΡΉι≥…œύΆ§Θ§ΕΦΚ§90%ΒΡΧΦΘ§ΧΰAΕ‘«βΤχΒΡœύΕ‘ΟήΕ» «20ΘΜΧΰB ΫΝΩ «ΧΰAΒΡ3±ΕΘ§ΧΰA‘Ύ“ΜΕ®ΧθΦΰœ¬Ρή”κΉψΝΩΒΡCl2ΤπΦ”≥…Ζ¥”ΠΘ§…ζ≥…1Θ§1Θ§2Θ§2Θ≠ΥΡ¬»±ϊΆιΘ§ΧΰB «±ΫΒΡΆ§œΒΈοΘ§Β±Υϋ”κCl2ΖΔ…ζ»Γ¥ζΖ¥”Π ±Θ®»Γ¥ζ±ΫΜΖ…œΒΡH‘≠Ή”Θ©Θ§…ζ≥…ΒΡ“Μ¬»¥ζΈοΓΔΕ଻¥ζΈοΓΔ»ΐ¬»¥ζΈοΖ÷±πΕΦ÷Μ”–“Μ÷÷Θ§ΗυΨί“‘…œ Β―ι ¬ ΒΘ§ΆΤΕœAΓΔBΝΫΧΰΒΡΖ÷Ή” ΫΓΔΫαΙΙΦρ ΫΚΆΟϊ≥ΤΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com