
·ÖĪö £Ø1£©ŅĄ¾ŻČČ»Æѧ·½³ĢŹ½½įŗĻøĒĖ¹¶ØĀɼĘĖćµĆµ½£»
£Ø2£©øł¾ŻČČ»Æѧ·½³ĢŹ½µÄŹéŠ“·½·ØæÉÖŖ£¬ĪļÖŹµÄĪļÖŹµÄĮæÓė·“Ó¦·Å³öµÄČČĮæ³ÉÕż±Č£¬²¢×¢Ņā±źĆ÷ø÷ĪļÖŹµÄ¾Ū¼ÆדĢ¬Ą“½ā“š£»
ĻČøł¾ŻøĒĖ¹¶ØĀÉŠ“³ö·½³ĢŹ½£¬Č»ŗóøł¾ŻĪļÖŹµÄĪļÖŹµÄĮæÓė·“Ó¦·Å³öµÄČČĮæ³ÉÕż±ČĄ“½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¢ŁC3H8£Øg£©=CH4£Øg£©+HC”ŌCH£Øg£©+H2£Øg£©”÷H1=+156.6kJ•mol-1
¢ŚCH3CH=CH2£Øg£©=CH4£Øg£©+HC”ŌCH£Øg £©”÷H2=+32.4kJ•mol-1
ŅĄ¾ŻøĒĖ¹¶ØĀÉ¢Ł-¢ŚµĆµ½ČČ»Æѧ·½³ĢŹ½ĪŖ£ŗC3H8£Øg£©=CH3CH=CH2£Øg£©+H2£Øg£©”÷H=+124.2 kJ•mol-1 £»
¹Ź“š°øĪŖ£ŗC3H8£Øg£©=CH3CH=CH2£Øg£©+H2£Øg£©”÷H=+124.2 kJ•mol-1 £»
£Ø2£©0.3molĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³ö649.5KJµÄČČĮ棬Ōņ1molĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³ö2165KJµÄČČĮ棬·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£¬
øł¾Ż£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£» ¢Ł
H2O£Øl£©”śH2O£Øg£©£»”÷H=+44kJ/moL£¬¢Ś
¢Ł+¢Ś”Į3µĆ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øg£©”÷H=-2033kJ/mol£¬¹Ź5.6L£Ø±ź×¼×“æö£©¼“0.25molŅŅÅšĶéĶźČ«Č¼ÉÕÉś³ÉĘųĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒĪŖ2033kJ”Į0.25=508.25kJ£»
¹Ź“š°øĪŖ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£»508.25 kJ£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖČČ»Æѧ·½³ĢŹ½µÄŹéŠ“£¬øĒĖ¹¶ØĀɵÄŌĖÓĆŅŌ¼°ČČĮæµÄ¼ĘĖć£¬ŠčŅŖ×¢ŅāµÄŹĒ·“Ó¦ČȵďżÖµÓė»Æѧ·½³ĢŹ½Ē°ĆęµÄĻµŹż³ÉÕż±Č£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
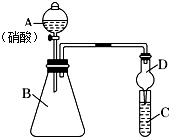 ѧĻ°ĮĖŌŖĖŲÖÜĘŚĀɵÄÓŠ¹ŲÖŖŹ¶ŗó£¬Ä³Ķ¬Ń§øł¾ŻŌŖĖŲ·Ē½šŹōŠŌÓė¶ŌÓ¦×īøß¼Ūŗ¬ŃõĖįÖ®¼äµÄ¹ŲĻµ£¬Ń”ŌńÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļÉč¼ĘĮĖČēĶ¼1×°ÖĆĄ“Ņ»“ĪŠŌĶź³ÉĶ¬Ö÷×åŗĶĶ¬ÖÜĘŚŌŖĖŲ·Ē½šŹōŠŌĒæČõ±Č½ĻµÄŹµŃéŃŠ¾æ£®
ѧĻ°ĮĖŌŖĖŲÖÜĘŚĀɵÄÓŠ¹ŲÖŖŹ¶ŗó£¬Ä³Ķ¬Ń§øł¾ŻŌŖĖŲ·Ē½šŹōŠŌÓė¶ŌÓ¦×īøß¼Ūŗ¬ŃõĖįÖ®¼äµÄ¹ŲĻµ£¬Ń”ŌńÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļÉč¼ĘĮĖČēĶ¼1×°ÖĆĄ“Ņ»“ĪŠŌĶź³ÉĶ¬Ö÷×åŗĶĶ¬ÖÜĘŚŌŖĖŲ·Ē½šŹōŠŌĒæČõ±Č½ĻµÄŹµŃéŃŠ¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
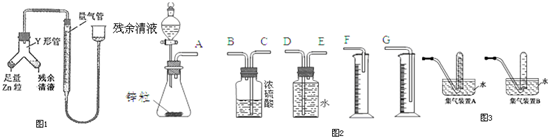
 ŹµŃéŹŅŠčŅŖ0.1mol/LNaOHČÜŅŗ500mL£¬øł¾ŻÕāÖÖČÜŅŗµÄÅäÖĘĒéæö»Ų“šĻĀĮŠĪŹĢā£®
ŹµŃéŹŅŠčŅŖ0.1mol/LNaOHČÜŅŗ500mL£¬øł¾ŻÕāÖÖČÜŅŗµÄÅäÖĘĒéæö»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
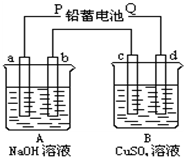 ÓĆČēĶ¼×°ÖĆŹµŃ飬A”¢BĮ½ÉÕ±·Ö±šŹ¢·Å200g10%NaOHŗĶ×ćĮæCuSO4ČÜŅŗ£®ĶصēŅ»¶ĪŹ±¼äŗó£¬c¼«ÉĻÓŠCuĪö³ö£¬ÓÖ²āµĆA±ÖŠČÜŅŗµÄÖŹĮæ¼õÉŁ4.5g£Ø²»æ¼ĀĒĖ®µÄÕō·¢£©£®
ÓĆČēĶ¼×°ÖĆŹµŃ飬A”¢BĮ½ÉÕ±·Ö±šŹ¢·Å200g10%NaOHŗĶ×ćĮæCuSO4ČÜŅŗ£®ĶصēŅ»¶ĪŹ±¼äŗó£¬c¼«ÉĻÓŠCuĪö³ö£¬ÓÖ²āµĆA±ÖŠČÜŅŗµÄÖŹĮæ¼õÉŁ4.5g£Ø²»æ¼ĀĒĖ®µÄÕō·¢£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 98 g H2SO4 | B£® | 56 g Fe | C£® | 44.8 L HCl | D£® | 6 g H2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ³ĀŹö¢ń | ³ĀŹö¢ņ | ÅŠ¶Ļ |
| A | Ģ¼ĖįÄĘČÜŅŗæÉÓĆÓŚÖĪĮĘĪø²” | Na2CO3æÉÓėŃĪĖį·“Ó¦ | ¢ń¶Ō£¬¢ņ¶Ō£¬ÓŠ |
| B | ĻņNa2O2µÄĖ®ČÜŅŗÖŠµĪČė·ÓĢŖ±äŗģÉ« | Na2O2ÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆÄĘ | ¢ń¶Ō£¬¢ņ“ķ£¬ĪŽ |
| C | ½šŹōÄʱ£“ęŌŚĆŗÓĶÖŠ£¬ŅŌøō¾ųæÕĘų | ³£ĪĀĻĀ£¬½šŹōÄĘŌŚæÕĘųÖŠ»įÉś³É¹żŃõ»ÆÄĘ | ¢ń¶Ō£¬¢ņ¶Ō£¬ÓŠ |
| D | ¹żŃõ»ÆÄĘæÉÓĆÓŚŗ½ĢģŌ±µÄ¹©Ńõ¼Į | Na2O2ÄÜŗĶCO2ŗĶH2O·“Ӧɜ³ÉO2 | ¢ń¶Ō£¬¢ņ¶Ō£¬ÓŠ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪļÖŹ | A | B | C | D |
| ĘšŹ¼Ķ¶ĮĻ/mol | 2 | 1 | 2 | 0 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com