
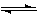
 CH3OH(g) ��H����a kJ��mol��1
CH3OH(g) ��H����a kJ��mol��1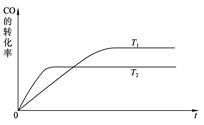
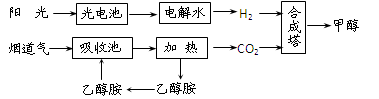
 HOCH2CH2NH3���� OH����2�֣�
HOCH2CH2NH3���� OH����2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����NaHSO4�����������ʱ�����ֻ�ѧ������ |
| B��ˮ���Ӽ���Ϊ�������,���Լ������ϸ��¶�ʱҲ���Էֽ� |
| C�����ݽ�������Ĺ��Կ�֪�����������Ӽ�һ��û�з����Ժͱ����� |
| D���Լ��Թ��ۼ��γɵķ���һ���Ǽ��Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢڢ� | B���ۢ٢� | C���ڢ٢� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����κ����ʷ����ж����л�ѧ�� |
| B��HF��HCl��HBr��HI�ķе��������� |
| C��D2O������H2O����������������������ȵķ��� |
| D��CO2��PCl3����������ԭ�Ӷ����������8�����ȶ��ṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڵ��ʵľ�����һ�����������Ӽ� |
| B�����Ӿ�����ֻ���ڷ��Ӽ���������������������ѧ�� |
| C���κξ����У�������������Ҳһ������������ |
| D�������ڳ����¶��Ծ�����ʽ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com