
| A������ | B������ | C������ | D���ؽᾧ��E��������F��������G����⡡H�����ȷֽ⡡I������J���������� |
 ����֬������Ӧ�Ļ��Һ�з������֬������________��
����֬������Ӧ�Ļ��Һ�з������֬������________�� ����________��
����________�� aCl________��
aCl________�� �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
g2����Ba2����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺 ��ԭ��Һ��һ�������ڵ�������_______________________��
��ԭ��Һ��һ�������ڵ�������_______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢܢ� | B���ۢ� | C���ۢܢ� | D���ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢݢ� |
| C���ۢ� | D���ޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
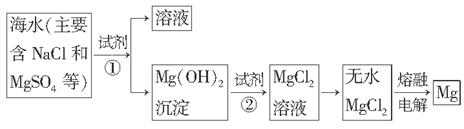
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 �ⶨ������������
�ⶨ������������ �ⶨʣ����������
�ⶨʣ����������| A����ҺAѡ��NaOH��Һ |
| B������ҺBѡ��Ũ���ᣬ����þ����������ƫС |
| C����ҺA��B����ѡ��ϡ���� |
| D��ʵ�����з����������ʵʩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢܢݢޢ� | B���٢ڢۢܢݢ� | C���٢ۢܢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������� | B��ʯ����Һ | C����ˮ | D������Cu(OH)2����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaCl�к�������Na2SO4���Ȼ����� |
| B��Fe SO4�к�������CuSO4�����ۣ� |
| C��CO2�к�������HCl���壨����������Һ�� |
| D��CO2�к���������CO�����ȵ�����ͭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com