
 A12O3+2Fe£¬
A12O3+2Fe£¬ A12O3+2Fe£»
A12O3+2Fe£» 2Fe2O3+4SO2£»
2Fe2O3+4SO2£» 2Fe2O3+4SO2 £»
2Fe2O3+4SO2 £»

 ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
ŠĀæĪ±ź½×ĢŻŌĶĮѵĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
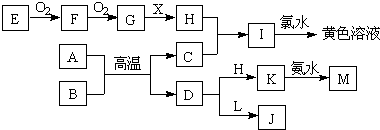
£Ø12·Ö£©ĻĀĮŠæņĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬A”¢B”¢C”¢D”¢E¶¼ŹĒ³£¼ūŌŖĖŲµÄµ„ÖŹ£¬ŌŚ³£ĪĀ³£Ń¹ĻĀAŹĒ¹ĢĢ壬ĘäÓą¶¼ŹĒĘųĢ壬ĒŅC³Ź»ĘĀĢÉ«”£»ÆŗĻĪļHŗĶIĮ½ÖÖĘųĢåĻąÓöŹ±²śÉś°×ŃĢ”£»ÆŗĻĪļGµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«”£Ķس£ĒéæöĻĀLŹĒĪŽÉ«ŅŗĢ唣·“Ó¦¢ŁŗĶ¢Ś¾łŌŚČÜŅŗÖŠ½ųŠŠ”£
Ēė°“ŅŖĒóĪŹ“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗF ”¢K ”£
£Ø2£©Čō·“Ó¦¢ŁŌŚČÜŅŗÖŠ½ųŠŠ£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©ŹµŃéŹŅÖĘČ”IµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻņJČÜŅŗÖŠµĪČėNaOHČÜŅŗŹ±£¬ĻÖĻóĪŖ £¬ÓĆ»Æѧ·½³ĢŹ½½āŹĶøƱä»Æ¹ż³Ģ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğøŹĖąĄ¼ÖŻŅ»ÖŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ĻĀĮŠæņĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬A”¢B”¢C”¢D”¢E¶¼ŹĒ³£¼ūŌŖĖŲµÄµ„ÖŹ£¬ŌŚ³£ĪĀ³£Ń¹ĻĀAŹĒ¹ĢĢ壬ĘäÓą¶¼ŹĒĘųĢ壬ĒŅC³Ź»ĘĀĢÉ«”£»ÆŗĻĪļHŗĶIĮ½ÖÖĘųĢåĻąÓöŹ±²śÉś°×ŃĢ”£»ÆŗĻĪļGµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«”£Ķس£ĒéæöĻĀLŹĒĪŽÉ«ŅŗĢ唣·“Ó¦¢ŁŗĶ¢Ś¾łŌŚČÜŅŗÖŠ½ųŠŠ”£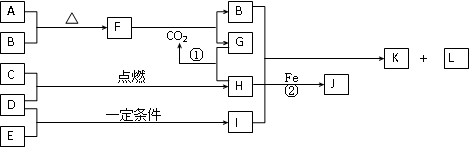
Ēė°“ŅŖĒóĪŹ“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗF ”¢K ”£
£Ø2£©Čō·“Ó¦¢ŁŌŚČÜŅŗÖŠ½ųŠŠ£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©ŹµŃéŹŅÖĘČ”IµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻņJČÜŅŗÖŠµĪČėNaOHČÜŅŗŹ±£¬ĻÖĻóĪŖ £¬ÓĆ»Æѧ·½³ĢŹ½½āŹĶøƱä»Æ¹ż³Ģ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģøŹĖąĄ¼ÖŻŅ»ÖŠøßŅ»ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ĻĀĮŠæņĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµÖŠ£¬A”¢B”¢C”¢D”¢E¶¼ŹĒ³£¼ūŌŖĖŲµÄµ„ÖŹ£¬ŌŚ³£ĪĀ³£Ń¹ĻĀAŹĒ¹ĢĢ壬ĘäÓą¶¼ŹĒĘųĢ壬ĒŅC³Ź»ĘĀĢÉ«”£»ÆŗĻĪļHŗĶIĮ½ÖÖĘųĢåĻąÓöŹ±²śÉś°×ŃĢ”£»ÆŗĻĪļGµÄŃęÉ«·“Ó¦ĪŖ»ĘÉ«”£Ķس£ĒéæöĻĀLŹĒĪŽÉ«ŅŗĢ唣·“Ó¦¢ŁŗĶ¢Ś¾łŌŚČÜŅŗÖŠ½ųŠŠ”£

Ēė°“ŅŖĒóĪŹ“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗF ”¢K ”£
£Ø2£©Čō·“Ó¦¢ŁŌŚČÜŅŗÖŠ½ųŠŠ£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø3£©ŹµŃéŹŅÖĘČ”IµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻņJČÜŅŗÖŠµĪČėNaOHČÜŅŗŹ±£¬ĻÖĻóĪŖ £¬ÓĆ»Æѧ·½³ĢŹ½½āŹĶøƱä»Æ¹ż³Ģ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com