

| ���� |
| ���� |
| ���� |
| ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
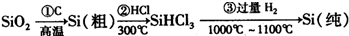
| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2012�������ѧһ�ָ���ѵ������3�¡���6�����ȼ��仯����(³�ư�) ���ͣ�043
ij������������Ư�ۣ���Ʒ˵�������£�

����ش�
(1)����ΪƯ��дһ�ݱ��桢ʹ�õ�ע������(������30��)��
________��
(2)�û�ѧ����ʽ��ʾ��
��Ư�۵�����ԭ����________��
��һ������£�Ư��ʧЧ����Ҫԭ��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com