
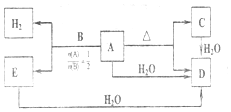
 ��
������ Q��R��T��Z��Y��X��Ϊ������Ԫ�أ����������μ��٣�����ֻ��T��XΪ������
��Zԭ�������������Ǵ�����3�����ж�ΪO��
��Q��T��ԭ������������֮��Ϊ10��ZΪOԪ�أ���QΪCl��TΪAl��
��Q��R���γ���ͬ��������Ų��������ӣ�����������仯�õ�RΪS��
��R��Zԭ��������������ͬ��
��R��Z���γ���ԭ�Ӻ�50���ӵĸ�����������L2-����������ӵ��������õ�������ΪSO42-��
��1��Q��T������������ˮ�������ӦΪ��������Һ������������Һ�ķ�Ӧ�����ɸ���������ˮ��
��2��R��T��Z�γɵĻ�����Ϊ�������������Ӽ����ۼ���
��3��BΪXH��B��H���⣩����������Ϊ12.50%����$\frac{1}{X+1}$=0.125��X=7����XĦ������Ϊ7���ж�ΪLi��D���ܶ�Ϊ0.7589g/L����״��������ɫ���壬��Ħ������=0.7589g/L��22.4L/mol=17g/mol�����Ȼ����������ɰ��̣��ж�DΪNH3��ת����ϵ��A���ȷֽ����ɰ�����C��2LiNH2$\frac{\underline{\;\;��\;\;}}{\;}$NH3+Li2NH�����ԭ���غ�õ�CΪLi2NH��AΪLiNH2��BΪLiH��Ϸ���������ԭ��Ӧ����������EΪLi3N���ƶ�YΪN��
��CΪLi2NH��DΪ����Ϊ���ۻ����Nԭ�ӵ��������5�����ӣ�����3��δ�ɶԵ��Ӻ�1�ԳɶԵ��ӣ�
��T����ΪAl3+��Z����ΪO2-��Y����ΪN3-�����Ӳ�����ͬ�����Ӱ뾶��˵���������С��
��E+H2O��D��Ӧ��Li3N��ˮ��Ӧ���ɰ�����������ﮣ�
������������Һ��ͨ�����D�õ���ɫ����Һ��������Һ��
��G����H���⣩��Y��T��R��Z�γɵĸ��Σ������������Ӻ�һ��������L2-����Ϊ��NH4��Al��SO4��2��0.01molG����Һ�м���0.024molBa��OH��2��������ӷ�Ӧ�Ķ�����ϵ�������ɳ��������ʵ�����
��� �⣺Q��R��T��Z��Y��X��Ϊ������Ԫ�أ����������μ��٣�����ֻ��T��XΪ������
��Zԭ�������������Ǵ�����3�����ж�ΪO��
��Q��T��ԭ������������֮��Ϊ10��ZΪOԪ�أ���QΪCl��TΪAl��
��Q��R���γ���ͬ��������Ų��������ӣ�����������仯�õ�RΪS��
��R��Zԭ��������������ͬ��
��R��Z���γ���ԭ�Ӻ�50���ӵĸ�����������L2-����������ӵ��������õ�������ΪSO42-��
��1��Q��T������������ˮ�������ӦΪ��������Һ������������Һ�ķ�Ӧ�����ɸ���������ˮ����Ӧ�Ļ�ѧ����ʽΪ��3HClO4+Al��OH��3=Al��ClO4��3+3H2O��
�ʴ�Ϊ��3HClO4+Al��OH��3=Al��ClO4��3+3H2O��
��2��R��T��Z�γɵĻ�����Ϊ�������������Ӽ����ۼ����ʴ�Ϊ�����Ӽ����ۼ���
��3��BΪXH��B��H���⣩����������Ϊ12.50%����$\frac{1}{X+1}$=0.125��X=7����XĦ������Ϊ7���ж�ΪLi��D���ܶ�Ϊ0.7589g/L����״��������ɫ���壬��Ħ������=0.7589g/L��22.4L/mol=17g/mol�����Ȼ����������ɰ��̣��ж�DΪNH3��ת����ϵ��A���ȷֽ����ɰ�����C��2LiNH2$\frac{\underline{\;\;��\;\;}}{\;}$NH3+Li2NH�����ԭ���غ�õ�CΪLi2NH��AΪLiNH2��BΪLiH��Ϸ���������ԭ��Ӧ����������EΪLi3N��
��CΪLi2NH��Nԭ�ӵ��������5�����ӣ�����3��δ�ɶԵ��Ӻ�1�ԳɶԵ��ӣ�3��δ�ɶԵ��ӷֱ��3��Hԭ���γ�3�Թ��õ��Ӷԣ��ʰ����ĵ���ʽΪ ��
��
�ʴ�Ϊ��Li2NH�� ��
��
��T����ΪAl3+��Z����ΪO2-��Y����ΪN3-�����Ӳ�����ͬ�����Ӱ뾶��˵���������С��T��Z��Y�����Ӱ뾶�ɴ�С��˳��Ϊ��N3-��O2-��Al3+��
�ʴ�Ϊ��N3-��O2-��Al3+��
��E+H2O��D��Ӧ��Li3N��ˮ��Ӧ���ɰ�����������ﮣ���Ӧ�Ļ�ѧ����ʽΪ��Li3N+3H2O=3LiOH+NH3����
�ʴ�Ϊ��Li3N+3H2O=3LiOH+NH3����
������������Һ��ͨ�����D�õ���ɫ����ҺΪ������Һ���÷�Ӧ�����ӷ���ʽΪ��Ag++2NH3•H2O=Ag��NH3��2++2H2O��
�ʴ�Ϊ��Ag++2NH3•H2O=Ag��NH3��2++2H2O��
��G����H���⣩��Y��T��R��Z�γɵĸ��Σ������������Ӻ�һ��������L2-����Ϊ��NH4��Al��SO4��2��0.01molG����Һ�м���0.024molBa��OH��2��G��Һ�к���n��NH4+��=0.01mol��n��Al3+��=0.01mol��n��SO42-��=0.04mol��0.024molBa��OH��2��Һ��n��Ba2+��=0.024mol��n��OH-��=0.048mol��
������ӦΪ��Al3++3OH-=Al��OH��3����
0.01mol 0.03mol 0.01mol
Ba2++SO42-=BaSO4����
0.024mol 0.024mol 0.024mol
NH4++OH-=NH3•H2O
0.01mol 0.01mol
ʣ���������������ʵ���=0.048mol-0.03mol-0.01mol=0.008mol
Al��OH��3+OH-=AlO2-+2H2O
0.008mol 0.008mol
ʣ�������������ʵ���=0.01mol-0.008mol=0.002mol��
���õ��������ʵ���=0.002mol+0.024mol=0.026mol��
�ʴ�Ϊ��0.026mol��
���� ���⿼����ԭ�ӽṹ�����ڱ����ɷ���Ӧ�á�������ɽṹ���жϣ���ѧʽ�ļ��㡢��ѧ����ʽ������ϵ����ȣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ܷ���������Ӧ���ܷ���ˮ�ⷴӦ | |
| B�� | 1mol �����������뺬2mol�嵥�ʵ�Ũ��ˮ��Ӧ | |
| C�� | 1mol ������������4molNaOH��Ӧ | |
| D�� | ��Na��NaHCO3��Na2CO3���ܷ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
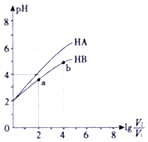 ��25���£�ϡ��HA��HB��������Һ��ŨҺpH�仯��������ͼ��ʾ������V1��ʾϡ��ǰ��������V2��ʾϡ�ͺ���Һ�����������˵������ȷ���ǣ�������
��25���£�ϡ��HA��HB��������Һ��ŨҺpH�仯��������ͼ��ʾ������V1��ʾϡ��ǰ��������V2��ʾϡ�ͺ���Һ�����������˵������ȷ���ǣ�������| A�� | a��b����ˮ�ĵ���̶�aС��b | |
| B�� | HA��HB��������ҺpH��ͬʱ��c��HA����c��HB�� | |
| C�� | ������a��b����$\frac{c��{B}^{-}��}{c��HB��•��O{H}^{-}��}$һ����� | |
| D�� | 25��ʱ��NaA��Һ��c��A-��һ������NaB��Һ��c��B-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1molHCl���� | B�� | 0.1molNa2SO4���� | ||
| C�� | 0.2molNaOH���� | D�� | 0.1mol�����Ǿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʽ����C4H8��ͬ���칹����4�� | |
| B�� | 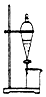 ����ͼ��ʾװ�������ˮ��ȥӲ֬�����еĸ��� | |
| C�� | �Ҵ��ķе���ڱ��飬�����ķе�������⣬������Ϊ���Ӽ������� | |
| D�� | �����ж�����Ƥ���и�ʴ�ԣ�������մ��Ƥ���ϣ�Ӧ������ˮϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ��Ҫ�� | ��ѧ�Լ� |
| ��1������ƾ����Ƿ���ˮ | �� |
| ��2������CO�е�H2O | �� |
| ��3����֤ζ�����Ƿ���ʳ�� | �� |
| ��4�������ˮ�еĵ� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����£��������pH��Ϊ3��HA��HB��Һ�ֱ��ˮϡ�ͣ���ҺpHֵ�ı仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����£��������pH��Ϊ3��HA��HB��Һ�ֱ��ˮϡ�ͣ���ҺpHֵ�ı仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��HB��Һ�еμ�NaOH��Һ�Ĺ����У�ˮ�ĵ���̶�һֱ���� | |
| B�� | ��pHΪ11��ij����pHΪ3��HB��Һ�������Ϻ���Һ�������Լ��� | |
| C�� | ��ˮ��HA��Һ��Ϻ����Һ�п��ܴ��ڣ�c��NH4+����c��A-����c��H+����c��OH-�� | |
| D�� | ��ȫ�к͵������pH��HA��HB����Һʱ������ͬŨ��NaOH��Һ�������HA��HB |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����AgBr��AgI���������Һ��c��Ag+����c��Br-��=c��I-�� | |
| B�� | 25��ʱ��0.1mol•L-1������ҺPH=a��0.01mol•L-1������ҺPH=b����b=a+1 | |
| C�� | 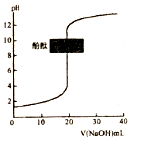 �����£�ͼ��ʾ�Է�̪��ָʾ������0.1mol•L-1NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�ζ����յ�ʱ����Һһ�������� | |
| D�� | ��0.1mol•L-1�İ�ˮ�м�������粒��壬����Һ��c��OH-��/c��NH3•H2O����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ҷ�αӫ����ī����������֬�����ϵ��Ƴɣ�������֬�����л��߷��Ӳ��� | |
| B�� | ���ع��͡���ֹʳ�ã������������Ʒ�����Ҳ����ͨ��������ȡ�õ����� | |
| C�� | ���������������Ӿ������������γ� | |
| D�� | NH4HCO3�����ֽܷ�������壬��ʳƷ��ҵ�п���Ϊ���Ƹ������ɼ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com