
(18��)ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�顣
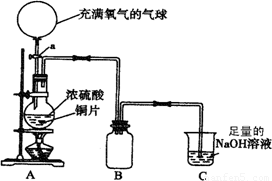
��1���ȹرջ���a����6.4gͭƬ��12 mLijŨ�ȵ�Ũ�������Բ����ƿ�й�������Ӧ��ϣ�������ƿ�л���ͭƬʣ�ࡣ�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
��д��������������ƿ�ڷ����Ļ�ѧ����ʽ���رջ���a ��
����a ��
��B�������ռ�ʵ���в����������װ�ã�������δ��ȫ��������ͼ�аѵ��ܲ���������
��ʵ�������װ��C�е���Һ�п��ܺ��е������� ��
��2����С���ͬѧ�ԡ���μ���SO2�л�������CO2���������ܸ���Ȥ������A��ͭƬ����ľ̿�ۣ�����A��B֮������������װ�ã�
�Լ���a. NaOH��Һ b. Ʒ����Һ c. ����KMnO4��Һ d. Ca(OH)2��Һ
��ش�
�ٸ�ͬѧ��ʵ��װ��A�з����Ļ�ѧ����ʽ ��
����Ҫ�ﵽ��Ŀ�ģ������ڣ�(�����ṩ�Լ����)
D��� ��E��� ��F��� ��
��3����ʵ֤ʵ���ڣ�1����ͭƬ��ȫ��ʧ����������ʣ�࣬��ͬѧ���ⶨ��������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����仯���Բ��ƣ�����Ӧ����Һ�м��뺬����a mol��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L(�ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(18��)ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�顣
��1���ȹرջ���a����6.4gͭƬ��12 mLijŨ�ȵ�Ũ�������Բ����ƿ�й�������Ӧ��ϣ�������ƿ�л���ͭƬʣ�ࡣ�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
��д��������������ƿ�ڷ����Ļ�ѧ����ʽ���رջ���a ��
����a ��
��B�������ռ�ʵ���в����������װ�ã�������δ��ȫ��������ͼ�аѵ��ܲ���������
��ʵ�������װ��C�е���Һ�п��ܺ��е������� ��
��2����С���ͬѧ�ԡ���μ���SO2�л�������CO2���������ܸ���Ȥ������A��ͭƬ����ľ̿�ۣ�����A��B֮������������װ�ã�
�Լ���a. NaOH��Һ b. Ʒ����Һ c. ����KMnO4��Һ d. Ca(OH)2��Һ
��ش�
�ٸ�ͬѧ��ʵ��װ��A�з����Ļ�ѧ����ʽ ��
����Ҫ�ﵽ��Ŀ�ģ������ڣ�(�����ṩ�Լ����)
D��� ��E��� ��F��� ��
��3����ʵ֤ʵ���ڣ�1����ͭƬ��ȫ��ʧ����������ʣ�࣬��ͬѧ���ⶨ��������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����仯���Բ��ƣ�����Ӧ����Һ�м��뺬����a mol��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L(�ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������������ʦ���и�һ��ѧ�����п��Ի�ѧ���� ���ͣ�ʵ����
(18��)ij��ѧ��һ������ȤС��Ϊ̽��ͭ������ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�顣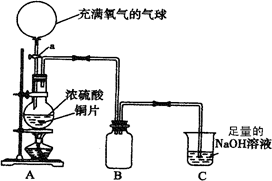
��1���ȹرջ���a����6.4gͭƬ��12 mLijŨ�ȵ�Ũ�������Բ����ƿ�й�������Ӧ��ϣ�������ƿ�л���ͭƬʣ�ࡣ�ٴ���a���������е�������������Բ����ƿ�����ͭƬ��ȫ��ʧ��
��д��������������ƿ�ڷ����Ļ�ѧ����ʽ���رջ���a ��
����a ��
��B�������ռ�ʵ���в����������װ�ã�������δ��ȫ��������ͼ�аѵ��ܲ���������
��ʵ�������װ��C�е���Һ�п��ܺ��е������� ��
��2����С���ͬѧ�ԡ���μ���SO2�л�������CO2���������ܸ���Ȥ������A��ͭƬ����ľ̿�ۣ�����A��B֮������������װ�ã�
�Լ���a. NaOH��Һ b. Ʒ����Һ c. ����KMnO4��Һ d. Ca(OH)2��Һ
��ش�
�ٸ�ͬѧ��ʵ��װ��A�з����Ļ�ѧ����ʽ  ��
��
����Ҫ�ﵽ��Ŀ�ģ������ڣ�(�����ṩ�Լ����)
D��� ��E��� ��F��� ��
��3����ʵ֤ʵ���ڣ�1����ͭƬ��ȫ��ʧ����������ʣ�࣬��ͬѧ���ⶨ��������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����仯���Բ��ƣ�����Ӧ����Һ�м��뺬����a mol ��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L(
��NaOH��Һ�պ�ʹ��Һ��Cu2+ȫ���������ݴˣ����������������ʵ���Ũ�������ܣ���д���������ʵ���Ũ�ȵı���ʽ�� mol/L( �ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��
�ú�a�Ĵ���ʽ��������ܣ��ÿղ���)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com