
����Ŀ��(1)������PbSO4����CH3COONH4��Һ���Ƶ�������ˮ��(CH3COO)2Pb�������ķ�ӦΪPbSO4��2CH3COONH4��(CH3COO)2Pb��(NH4)2SO4��˵��(CH3COO)2Pb��________(����ǿ����������)����ʡ�
(2)��֪������(H3PO2)������������������Һ��Ӧ����NaH2PO2��H2O�����������_____Ԫ��(����һ������������������)��
(3)��ҵ���Ʋ�����ˮ�����õ��Ĺ�ͬԭ����________(�ѧʽ)��
(4)��һ���¶��£���һ��2 L������ܱ�������(Ԥ��װ�����)ͨ��1 mol N2��3 mol H2��������Ӧ��N2(g)��3H2(g)2NH3(g)������һ��ʱ����������ѹǿ����ʼ��0.9�����ڴ�ʱ���ڣ�H2ƽ����Ӧ����Ϊ0.1 mol/(L��min)������������ʱ��Ϊ______min
(5)��������(Na2FeO4)����ǿ�����ԣ��ɶ�����ˮ�����������������������ƿ������������ʹ��������ڼ��Խ����з�Ӧ�õ����벹�䲢��ƽ�������ӷ���ʽ��
____Fe(OH)3 +____ClO��+____OH��=__FeO42����___Cl��+__
(6)�ڷ�Ӧ11P+15CuSO4+24H2O=5Cu3P+6H3PO4+15H2SO4�У���������___________������2mol H3PO4���ɣ�ת�Ƶĵ��ӵ����ʵ���Ϊ___________
���𰸡��� һ CaCO3 3 2 3 4 2 3 5H2O P��CuSO4 10mol
��������
(1)����Ǧ��ˮ��Һ������ܽ�ƽ�⣬��������ʱ����������Ӻ�Ǧ���������ѵ������ʻ�������ʴ���Ǧ����ʹ����Ǧ�ܽ⣬˵��(CH3COO)2Pb��������ʣ�
(2)H3PO2��������NaOH��Һ��Ӧ������NaH2PO2��˵��H3PO2ֻ�ܵ����һ�������ӣ�����H3PO2��һԪ�
(3)ˮ�������ԭ��Ϊ�����ʯ��ʯ���Ʋ�������Ҫԭ��Ϊ���ʯ��ʯ��ʯӢɰ����ͬԭ��ΪCaCO3��
(4)�������Ϊ2L�����Գ�ʼͶ��c(N2)=0.5mol/L��c(H2)=1.5mol/L�����c(N2)=x mol��������ʽ�У�
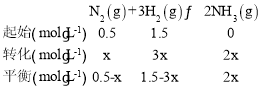
������ѹǿ����ʼ��0.9�����¶Ⱥ������ݻ����䣬�����ѹǿ�ȵ���Ũ��֮�ȣ�����![]() �����x=0.1mol/L�����c(H2)=0.3mol/L��v(N2)=0.1 mol/(L��min)����Ӧʱ��t=
�����x=0.1mol/L�����c(H2)=0.3mol/L��v(N2)=0.1 mol/(L��min)����Ӧʱ��t=![]() =3min��
=3min��
(5)��Ԫ�ػ��ϼ���+3����Ϊ+6��ʧȥ3�����ӣ���Ԫ�ػ��ϼ���+1����Ϊ-1����2�����ӣ�ȡ��С��������������������������ǰϵ��Ϊ2�����������������ǰϵ��Ϊ3���ٸ��ݵ���غ㼰ԭ���غ���ƽ��2Fe(OH)3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��
(6)Cu3P��PԪ�ػ��ϼ�Ϊ-3��H3PO4��PԪ�ػ��ϼ�Ϊ+5������P�������������ǻ�ԭ����CuԪ�ػ��ϼ���CuSO4�е�+2����ΪCu3P��+1������CuSO4Ҳ������������������ΪP��CuSO4������2molH3PO4���ɣ�ת�Ƶĵ��ӵ����ʵ���Ϊ2mol��(+5-0)=10mol��


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K3[Fe(C2O4)3]��3H2O�������������أ�Ϊ����ɫ���壬������ɹ����ͼ���ش��������⣺
��1��ɹ����ͼʱ����K3[Fe(C2O4)3]��3H2O���й������K3[Fe(CN)6]��ҺΪ��ɫ�������ⷴӦ�Ļ�ѧ����ʽΪ��2K3[Fe(C2O4)3]![]() 2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ijС��Ϊ̽�������������ص��ȷֽ�������ͼ��ʾװ�ý���ʵ�顣
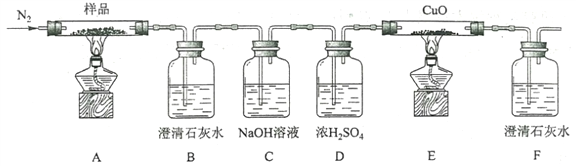
��ͨ�뵪����Ŀ����________________________________________��
��ʵ���й۲쵽װ��B��F�г���ʯ��ˮ������ǣ�װ��E�й����Ϊ��ɫ���ɴ��ж��ȷֽ������һ������___________��___________��
��Ϊ��ֹ������ֹͣʵ��ʱӦ���еIJ�����_____________________________��
����Ʒ��ȫ�ֽ��װ��A�еIJ����ﺬ��FeO��Fe2O3������Fe2O3���ڵķ����ǣ�________________��
��3���ⶨ�����������������ĺ�����
�ٳ���m g��Ʒ����ƿ�У��ܽ���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㡣�ζ��յ��������___________________________��
����������Һ�м������п������Ӧ��ȫ���ˡ�ϴ�ӣ�����Һ��ϴ��Һȫ���ռ�����ƿ�С���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㣬����KMnO4��ҺV mL���þ������������������ı���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��C3H6���ǻ����л�����ԭ�ϡ���A�Ʊ��ۺ���C��![]() �ĺϳ�·�ߣ����ַ�Ӧ������ȥ����ͼ��ʾ��
�ĺϳ�·�ߣ����ַ�Ӧ������ȥ����ͼ��ʾ��
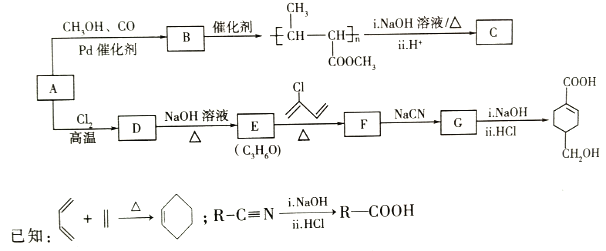
�ش��������⣺
��1��D��������_______��B���еĺ��������ŵ�������_______________��
��2��C�Ľṹ��ʽΪ_______________��D��E�ķ�Ӧ����Ϊ________________��
��3��E��F�Ļ�ѧ����ʽΪ____________________________��
��4��![]() �������__________��ԭ�ӹ�ƽ�棬
�������__________��ԭ�ӹ�ƽ�棬![]() �������۷�Ӧ�����л���Ľṹ��ʽΪ_______________��
�������۷�Ӧ�����л���Ľṹ��ʽΪ_______________��
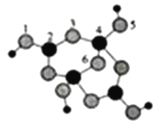
��5��B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����__________��д�ṹ��ʽ����
��6����������Ϣ������ϩ��HBrΪ��ʼԭ���Ʊ����ᣬ��ƺϳ�·�ߡ�(���Լ���ѡ���ϳ�·������ͼʾ�����������) ________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ�� Q��W��X��Y��Zԭ�������������ӣ�����Q��Wԭ�Ӻ���L ���Ӳ�ĵ������ֱ�Ϊ0��4��X��Y��Z�����ڱ��е�λ����ͼ��ʾ�� ����˵������ȷ����
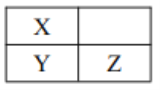
A. W�� X�� Q ��ԭ�Ӱ뾶���μ�С B. Y ������������ˮ����һ����ǿ��
C. W �� Z �����γɻ�����W3Z8 D. Q�� X�� Z �����γ����ӻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3��Һ�к��е�Mg2����K���������Ӳ������ܼ���AlCl3����ʧ��
 ������˵����ȷ����(����)
������˵����ȷ����(����)
A. NaOH��Һ�����ð�ˮ������
B. ��Һa�к���Al3����K����Cl����Na����OH��
C. ��Һb��ֻ����NaCl
D. ����Һa�еμ������������Һ��pH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ǧ��PbCrO4����һ��������ˮ�Ļ�ɫ���ϣ�����ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵��������ǣ� ��
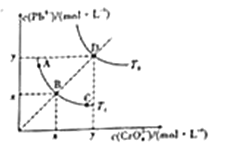
A. ͼ��x��y�ֱ�ΪT1��T2�¶���PbCrO4��ˮ�е��ܽ��
B. ͼ�и����Ӧ��Ksp�Ĺ�ϵΪKsp��A��=Ksp��C��<Ksp��B��<Ksp��D��
C. ��A�����Һ�м�������Na2CrO4���壬��Һ�����A��ABC����B�����ƶ�
D. �¶Ƚ���ʱ��D��ı�����Һ�������D��DB����B�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����⻯�ƣ�NaBH4���ڻ������������Ҫ��Ӧ�ü�ֵ���ɲ�����ɰ��SiO2��Na��H2��Ϊԭ���Ʊ����ش��������⣺
��1�����ڱ��У���B�Ļ�ѧ���������Ƶ�����Ԫ����____����Ԫ�ػ�̬ԭ�Ӻ���M�����������״̬��ͬ����_____����
��2��NaBH4�У��縺������Ԫ����____����Ԫ�ط��ţ���B��____�ӻ������H��1s����γ�![]() ����
����
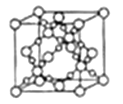
��3����ɰ�Ǻ�8���ᾧˮ���������ơ��������ӣ���B��O��H����Ԫ�أ�����ģ����ͼ��ʾ��
���������У���λ��������____��____ԭ��֮�䡣������ԭ�ӵ���ţ�
����ɰ�Ļ�ѧʽΪ_______��
��4��SiO2�����������壩��ͼ��ʾ����֪SiO2���ܶ�Ϊ![]() g/cm3���谢���ӵ�������ֵΪNA����SiO2�����ı߳�Ϊ___pm��
g/cm3���谢���ӵ�������ֵΪNA����SiO2�����ı߳�Ϊ___pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������װ���������ʵ�飨ͼ��a��b��c��d Ϊֹˮ�У���
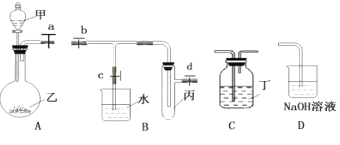
��1�������ҵ�����_________��
��2��װ�� A��B��D ��������֤��SO2��ˮ�е��ܽ�ȣ���ز��������ǣ���ȡ SO2���ռ� SO2�� �ر�ֹˮ�� b��d����ֹˮ�� c��_____________��
��3�����ձ����д���ˮ��������Թ��У�˵�� SO2 ������ˮ�� װ�� A��C ������������֤��C��Si �ķǽ�����ǿ�������Լ�����_____________��C �� �� �� �� ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɡ���ͬѧ�������ͼ1װ�ã�����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ������һ�������̼��Ԫ����C��Si�ķǽ�����ǿ���Ƚϵ�ʵ���о�����ͬѧ�������ͼ2װ������֤±��Ԫ�����ʵĵݱ���ɡ�A��B��C�����ֱ���պ��NaBr��Һ������ʪ��ĵ���KI��ֽ��պ�м�Һ��������֪������Ũ�������������ܷ�Ӧ����������
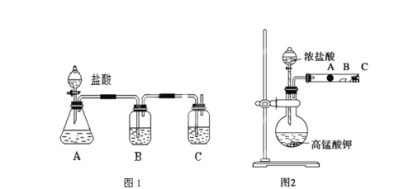
(1)����ʵ��ͼ1����
��д��ѡ���Լ��ֱ� B__________��C____________
��װ��B����ʢ�Լ�������Ϊ_________
��C�з�Ӧ�����ӷ���ʽΪ_______
�ܴ�ʵ�����÷ǽ�����C____Si(��������������С����)
(2)����ʵ��ͼ2����
��д�� A�������ӷ���ʽ��_______
��B��������_____
��C��ҩƷ������ _______��
��ͨ��ͼ2 װ��ʵ��ó�ͬ����Ԫ�����ʵĵݱ����Ϊ___________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com