��֪�ɶ�����Ԫ����ɵ�������A��B��C��D��E��F������ԭ�Ӻ���Ŀ����Ϊ2��3��4��5��1��1������A��B��E������18�����ӣ�C��D��F������10�����ӣ�A��B��C��DΪ�����������ش�
��1��D���ӵĿռ乹��Ϊ
��������
��������
��
��2����B����ͨ��Cu��OH��
2����Һ�У�����Һ��Ϊ��ɫ����Ӧ�Ļ�ѧ����ʽΪ
H2S+Cu��OH��2=CuS+2H2O
H2S+Cu��OH��2=CuS+2H2O
��
��3��A��C��Ͽ�����һ���µĻ�����û������к��еĻ�ѧ��������
���Ӽ������ۼ�
���Ӽ������ۼ�
�������£���pH=a��C��ϡ��Һ��pH=14-a��Aϡ��Һ�������ϣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊ
c��NH4+����c��Cl-����c��OH-����c��H+��
c��NH4+����c��Cl-����c��OH-����c��H+��
��
��4������E��F�ɰ�1��1������ӻ�����X����ˮ��Һ�����ԣ���д�����X��Һ�Ļ�ѧ����ʽ��
2NaCl+2H
2O
H
2��+Cl
2��+2NaOH
2NaCl+2H
2O
H
2��+Cl
2��+2NaOH
�����ʱ�������ռ������������С��������ԭ����
����Cl2��NaOH��Һ�����˷�Ӧ������
����Cl2��NaOH��Һ�����˷�Ӧ������
��
����Y��Һ���������Ӽ������¹�ϵ��c��H
+��+3c��F��=c��OH
-��+c��E������Y��Һ��pH
��
��
7������ڡ�����С�ڡ����ڡ�����ԭ���ǣ������ӷ���ʽ��ʾ��
Al3++3H2O?Al��OH��3+3H+
Al3++3H2O?Al��OH��3+3H+
��

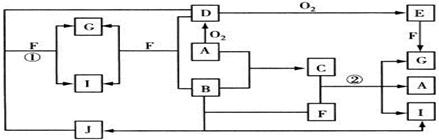




 ���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��
���ҿ�ͼ��Ӧ�����漰��l4�����ʶ����ɶ�����Ԫ����ɵģ���֪��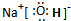
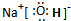
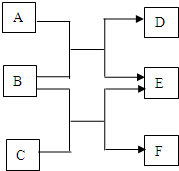 A��B��C��D��E��F�������ʾ����ɶ�����Ԫ����ɵ���ѧ�����Ļ�ѧ���ʣ�����֮���ת����ϵ����ͼ��
A��B��C��D��E��F�������ʾ����ɶ�����Ԫ����ɵ���ѧ�����Ļ�ѧ���ʣ�����֮���ת����ϵ����ͼ��

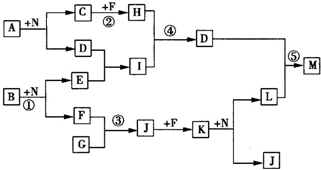
 �ס������ɶ�����Ԫ����ɵij������ʻ���������ѧ��ѧ�����������ʻ�����������ͼ�ת����ϵ������˵������ȷ���ǣ�������
�ס������ɶ�����Ԫ����ɵij������ʻ���������ѧ��ѧ�����������ʻ�����������ͼ�ת����ϵ������˵������ȷ���ǣ�������