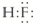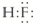��A��B��C��DΪǰ������Ԫ�ء�AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2��BԪ��ԭ�ӵ�����������������Ӳ�����3����CԪ��ԭ�ӵ�M�ܲ��p�ܼ���3��δ�ɶԵ��ӣ�DԪ��ԭ�Ӻ����M�ܲ���ֻ��2�ԳɶԵ��ӡ���ش��������⣺
�ŵ�n=2ʱ��AB2����_______���ӣ�����ԡ��Ǽ��ԡ�������������_______���м���A6H6������Aԭ�ӵ��ӻ����������_______�ӻ���
�Ƶ�n=3ʱ��A��B�γɵľ�������_______���塣
����AԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��A��C��D����Ԫ�صĵ�һ�������ɴ�С��˳����_______����Ԫ�ط��ű�ʾ����
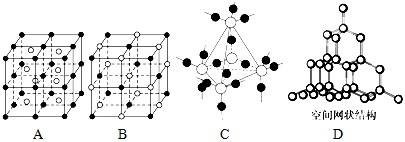
���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ��δ��˳����������ľ���������ͬ����__________(������Ӧ�ı����д)
�Ƣ���N
2O��Ϊ�ȵ������������Ϊ______________����һ�ּ��ɣ����ڸ��ݼ۲���ӶԻ���ģ��ȷ��ClO
3�����ӵĿռ乹��Ϊ______________��
����֪ij����ɫ���������ΪCoCl
3��5NH
3��H
2O����������е����������������ڻ�̬ʱ�ĺ�������Ų�ʽΪ___________��
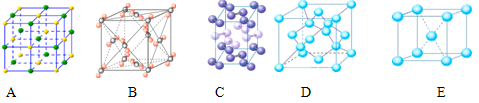
����ͼ�ǽ���������ṹ��ʾ��ͼ����֪���������ܶ�Ϊ2.7g/cm
3������ڵ���ԭ�ӵİ뾶Ϊ_____________cm������֪
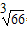
=4.04��

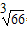 =4.04��
=4.04�� 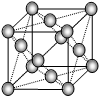

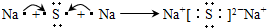
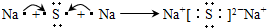
 ��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?
��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��? A��B��C��DΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���D����������Dԭ�Ӻ�������������������ȣ�
A��B��C��DΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���D����������Dԭ�Ӻ�������������������ȣ�