

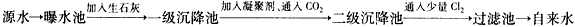

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| pHֵ | 6.5-8.5 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| PH | 6.5��8.5 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PH | 6.5��8.5 |
Ca2+��Mg2+��Ũ��/mol��L-1 | ��0.0045 |
ϸ������/����mol | ��100�� |
ͼ3-14��ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��
![]()
ͼ3-14
(1)ԭˮ�к�Ca2+��Mg2+��![]() ��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ__________________________________________________��
��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2�������������ɸ��ֽⷴӦ��д������һ�����ӷ���ʽ__________________________________________________��
(2)���ۼ���ȥ������������Ĺ���___________________________��(��д��ţ���ѡ����)
��ֻ���������� ��ֻ�ǻ�ѧ���� ���������ͻ�ѧ����
FeSO4��7H2O�dz��õ����ۼ�������ˮ����������___________________________������
(3)ͨ�������̼��Ŀ����__________________��____________________��
(4)����A��������___________�����������ǻ�������A��ˮ��Ӧ�IJ������________�ԡ�
(5)���������У�______________������Ϊ����A�Ĵ���Ʒ��(��д��ţ���ѡ����)
��Ca(ClO)2 ��Ũ��ˮ ��K2FeO4 ��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��涨����ˮ�����������������Ҫ��pHΪ6.5��8.5��Ca2+��Mg2+��Ũ��С��0.004 5 mol��L-1��ϸ������С��100��ÿ��������ͼ��Դˮ����������ˮ�Ĺ�������ʾ��ͼ��

(1)Դˮ�к�Ca2+��Mg2+��![]() ��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2����������������ֽⷴӦ����д������������Ӧ�����ӷ���ʽ����__________________����__________________����_________________________________��
��Cl-�ȣ�����ʯ�Һ�����Ca(OH)2����������������ֽⷴӦ����д������������Ӧ�����ӷ���ʽ����__________________����__________________����_________________________________��
(2)���ۼ���ȥ������������Ĺ�����______________________ (�����)��
A.ֻ���������� B.ֻ�ǻ�ѧ���� C.�������ͻ�ѧ����
(3)�̷���ˮ����������______________________������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡЭ�������5�µڶ���������ѧ�Ծ��������棩 ���ͣ������
��1�����й��ڹ�ҵ����˵����ȷ���� ��������ţ�
A���ں����Ƽҵ�У����Ȼ�����Һ����ͨ������̼����ͨ����
B�������Ṥҵ���ϳɰ���ҵ�����Ṥҵ�У��Բ���ѭ���������ԭ��������
C�����ȼҵ�����۱����ӽ���Ĥ���������Һ�������
D����ҵ�ϲ��õ�������Ȼ����ķ�����ȡ������
E��ʯ���ѻ����ڻ�ѧ�仯����ҪĿ����Ϊ�˻�ö�����������̬��
��2���ҹ��涨����ˮ�������涨��������±���Ҫ��
|
pH |
Ca2+ ��Mg2+��Ũ�� |
ϸ������ |
|
6.5��8.5 |
�� 0.004 5 mol��L-1? |
��100����mL-1? |
������ԭˮ����������ˮ�Ĺ�������ʾ��ͼ��

��ԭˮ�к�Ca2+ ��Mg2+ ��HCO3- ��Cl-�ȣ�����ʯ������Ca(OH)2�������������ɸ��ֽⷴӦ��д�����е����ӷ���ʽ��ֻҪ��д���������� �� ��
��FeSO4��7H2O�dz��õ����ۼ�������ˮ���������� ������ͨ�������̼��Ŀ���� �� ��
������A�������� ������������ ������Ϊ����A�Ĵ���Ʒ�����ţ���
a��ClO2 b��Ũ��ˮ c��K2FeO4 d��SO2 e.Ư��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com