
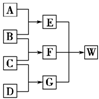
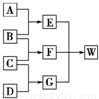
 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���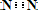 ��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3ΪNH3�����ӿռ�ṹΪ�������ͣ�
��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3ΪNH3�����ӿռ�ṹΪ�������ͣ�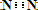 �������ͣ�
�������ͣ� 2NH3 ���ʴ�Ϊ��N2+3H2
2NH3 ���ʴ�Ϊ��N2+3H2 2NH3
2NH3 CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O�� CO2��+2SO2��+2H2O��
CO2��+2SO2��+2H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
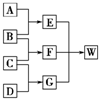
 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���

| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��֪EΪֱ���εķǼ��Է��ӣ���E�ĽṹʽΪ______________��D���ʵĵ���ʽΪ_______________��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3����G���ӵĿռ乹��Ϊ______________��
(2)C+D![]() G�Ļ�ѧ����ʽΪ__________________________________________��
G�Ļ�ѧ����ʽΪ__________________________________________��
(3)��֪������Ԫ��X��B�е�Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��Һ��A��Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(4)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��߿���ѧһ�ָ�ϰ������ѧ���뻯ѧ��Ӧ���������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com