
 �ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺
�ҹ����Ĵ���֮һ�ڻ�ҩ����ըʱ������Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C��K2S+3CO2��+N2�������������������Ԫ�ػش��������⣺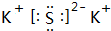 ���ʴ�Ϊ��O=C=O��
���ʴ�Ϊ��O=C=O��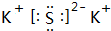 ��
��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
��S+Hg�T�THgS����3S+2Al![]() Al2S3��
Al2S3��
��S+2KNO3+3C![]() K2S+N2��+3CO2����
K2S+N2��+3CO2����
��3S+6KOH�T�T2K2S+K2SO3+3H2O�����Тٳ������������ڹ���������Ⱦ���������Ʊ�Al2S3�������ҹ��Ŵ��Ĵ���֮һ�����ڻ�ҩ��һ�������ľ̿���ı�ը��Ӧ��������ϴ�������Թܵ������ڱڵIJ�����ķ���֮һ��
�����ڷ�Ӧ�еļ�̬�仯���飬����________������������ͬ��������Ҫ�ķ���_______
_______________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�������������ĸ���Ӧ������������á�
��S+Hg�T�THgS����3S+2Al![]() Al2S3��
Al2S3��
��S+2KNO3+3C![]() K2S+N2��+3CO2����
K2S+N2��+3CO2����
��3S+6KOH�T�T2K2S+K2SO3+3H2O�����Тٳ������������ڹ���������Ⱦ���������Ʊ�Al2S3�������ҹ��Ŵ��Ĵ���֮һ�����ڻ�ҩ��һ�������ľ̿���ı�ը��Ӧ��������ϴ�������Թܵ������ڱڵIJ�����ķ���֮һ��
�����ڷ�Ӧ�еļ�̬�仯���飬����________������������ͬ��������Ҫ�ķ���_______
_______________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ������
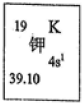
 �����б���ԭ��Ԫ����_________����������Ԫ����_________����������_________����ԭ����_________������������_________����ԭ������_________��
�����б���ԭ��Ԫ����_________����������Ԫ����_________����������_________����ԭ����_________������������_________����ԭ������_________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com