
 ĒāÄÜŹĒŅ»ÖÖ¼«¾ß·¢Õ¹Ē±Į¦µÄĒå½ąÄÜŌ“£®
ĒāÄÜŹĒŅ»ÖÖ¼«¾ß·¢Õ¹Ē±Į¦µÄĒå½ąÄÜŌ“£®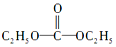 £¬ŌņĢ¼õ£ėĀµÄ½į¹¹¼ņŹ½ĪŖ
£¬ŌņĢ¼õ£ėĀµÄ½į¹¹¼ņŹ½ĪŖ £®
£® ·ÖĪö £Ø1£©SO2+2H2O+I2=H2SO4+2HIÖŠ¶žŃõ»ÆĮņÖŠĮņµÄ»ÆŗĻ¼ŪÉżøߏĒ»¹Ō¼Į£»¢ń+¢ņ+¢óµĆµāŃ»··Ö½āĖ®×Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½£»
£Ø2£©ŌŚøßĪĀĻĀ£¬N2H4æÉĶźČ«·Ö½āĪŖNH3”¢N2¼°H2£¬ĖłŅŌ·Ö½ā·“Ó¦·½³ĢŹ½ĪŖ£ŗ7N2H4$\frac{\underline{\;øßĪĀ\;}}{\;}$8NH3+3N2+2H2£»
£Ø3£©Ģ¼õ£ėĀ£ØĻą¶Ō·Ö×ÓÖŹĮæĪŖ90£©ŹĒÓÉDECÓėN2H4·¢ÉśČ”“ś·“Ó¦ÖĘµĆµÄ£¬ĖłŅŌĢ¼õ£ėĀµÄ½į¹¹¼ņŹ½ĪŖ £®
£®
½ā“š ½ā£ŗ£Ø1£©SO2+2H2O+I2=H2SO4+2HIÖŠ¶žŃõ»ÆĮņÖŠĮņµÄ»ÆŗĻ¼ŪÉżøߏĒ»¹Ō¼Į£»¢ń+¢ņ+¢óµĆµāŃ»··Ö½āĖ®×Ü·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬ĖłŅŌ·½³ĢŹ½ĪŖ£ŗ2H2O$\frac{\underline{“߻ƼĮ}}{”÷}$2H2”ü+O2”ü£¬¹Ź“š°øĪŖ£ŗSO2£»2H2O$\frac{\underline{“߻ƼĮ}}{”÷}$2H2”ü+O2”ü£»
£Ø2£©ŌŚøßĪĀĻĀ£¬N2H4æÉĶźČ«·Ö½āĪŖNH3”¢N2¼°H2£¬ĖłŅŌ·Ö½ā·“Ó¦·½³ĢŹ½ĪŖ£ŗ7N2H4$\frac{\underline{\;øßĪĀ\;}}{\;}$8NH3+3N2+2H2£¬¹Ź“š°øĪŖ£ŗ7N2H4$\frac{\underline{\;øßĪĀ\;}}{\;}$8NH3+3N2+2H2£»
£Ø3£©Ģ¼õ£ėĀ£ØĻą¶Ō·Ö×ÓÖŹĮæĪŖ90£©ŹĒÓÉDECÓėN2H4·¢ÉśČ”“ś·“Ó¦ÖĘµĆµÄ£¬ĖłŅŌĢ¼õ£ėĀµÄ½į¹¹¼ņŹ½ĪŖ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £®
£®
µćĘĄ ±¾Ģāæ¼²éŃõ»Æ»¹Ō·“Ó¦”¢»Æѧ·½³ĢŹ½µÄŹéŠ“”¢½į¹¹Ź½µÄĪŹĢāµČ£¬×ŪŗĻŠŌĒ棬µ«ÓŠŅ»¶ØµÄÄŃ¶Č£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«ĶĖæ²åČėĻ”ĻõĖįÖŠ£ŗCu+4H++2NO3-ØTCu2++2NO2”ü+2H2O | |
| B£® | ĻņFe2£ØSO4£©3ČÜŅŗÖŠ¼ÓČė¹żĮæĢś·Ū£ŗFe3++FeØT2Fe2+ | |
| C£® | ĻņAl2£ØSO4£©3ČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£ŗAl3++3NH3•H2OØTAl£ØOH£©3”ż+3NH4+ | |
| D£® | Ļ”H2SO4ÓėBa£ØOH£©2ČÜŅŗ·“Ó¦£ŗH++SO42-+Ba2++OH-ØTBaSO4”ż+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C+H2O$\frac{\underline{\;\;”÷\;\;}}{\;}$CO+H2 | B£® | H2+CuO$\frac{\underline{\;\;”÷\;\;}}{\;}$Cu+H2O | ||
| C£® | Cl2+H2O?HCl+HClO | D£® | SO3+H2O=H2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | .³£ĪĀ³£Ń¹ĻĀ£¬18 g H2OÖŠŗ¬ÓŠµÄµē×Ó×ÜŹżĪŖ10NA | |
| B£® | .1 mol•L-1 NaClČÜŅŗŗ¬ÓŠNAøöNa+ | |
| C£® | ³£ĪĀ³£Ń¹ĻĀ£¬92 gµÄNO2ŗĶN2O4»ģŗĻĘųĢåŗ¬ÓŠµÄŌ×ÓŹżĪŖ3 NA | |
| D£® | ±ź×¼×“æöĻĀ£¬2.24LCH4Ėłŗ¬ÖŠ×ÓŹżĪŖNA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ŅĶ„ÖŠ¾³£ÓĆŹ³“×½žÅŻÓŠĖ®¹øµÄĖ®ŗų | |
| B£® | øŹÓĶ¼ÓĖ®×÷»¤·ō¼Į | |
| C£® | ½»ĶؾƲģÓĆĖįŠŌÖŲøõĖį¼Ų¼ģ²éĖ¾»śŹĒ·ń¾Ęŗó¼Ż³µ | |
| D£® | ÅėÓ揱£¬¼ÓČėÉŁĮæµÄĮĻ¾ĘŗĶŹ³“×æɼõÉŁŠČĪ¶£¬Ōö¼ÓĻćĪ¶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗÓ±±Ź”ø߶žÉĻµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
½«1 mol I2£Øg£© ŗĶ2 mol H2ÖĆÓŚ2LĆܱÕČŻĘ÷ÖŠ£¬ŌŚŅ»¶ØĪĀ¶ČĻĀ·¢Éś·“Ó¦£ŗI2£Øg£© + H2£Øg£©  2HI£Øg£© ”÷H£¼0£¬²¢“ļĘ½ŗā”£HIµÄĢå»ż·ÖŹżw£ØHI£©Ėꏱ¼ä±ä»ÆČēĶ¼ĒśĻߣآņ£©ĖłŹ¾£ŗ
2HI£Øg£© ”÷H£¼0£¬²¢“ļĘ½ŗā”£HIµÄĢå»ż·ÖŹżw£ØHI£©Ėꏱ¼ä±ä»ÆČēĶ¼ĒśĻߣآņ£©ĖłŹ¾£ŗ
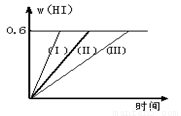
£Ø1£©“ļĘ½ŗāŹ±£¬I2£Øg£©µÄĪļÖŹµÄĮæÅضČĪŖmol/L ”£
£Ø2£©Čōøı䷓ӦĢõ¼ž£¬ŌŚ¼×Ģõ¼žĻĀw£ØHI£©µÄ±ä»ÆČēĒśĻߣآń£© ĖłŹ¾£¬ŌŚŅŅĢõ¼žĻĀw£ØHI£©µÄ±ä»ÆČēĒśĻß£Ø¢ó£© ĖłŹ¾”£Ōņ¼×Ģõ¼žæÉÄÜŹĒ £¬ŌņŅŅĢõ¼žæÉÄÜŹĒ ”££ØĢīČėĻĀĮŠĢõ¼žµÄŠņŗÅ£©
¢ŁŗćČŻĢõ¼žĻĀ£¬ÉżøßĪĀ¶Č£»¢ŚŗćČŻĢõ¼žĻĀ£¬½µµĶĪĀ¶Č£»¢ŪŗćĪĀĢõ¼žĻĀ£¬ĖõŠ”·“ӦȯĘ÷Ģå»ż£»¢ÜŗćĪĀĢõ¼žĻĀ£¬Ą©“ó·“ӦȯĘ÷Ģå»ż£»¢ŻŗćĪĀŗćČŻĢõ¼žĻĀ£¬¼ÓČėÕż“߻ƼĮ”£
£Ø3£©Čō±£³ÖĪĀ¶Č²»±ä£¬ŌŚĮķŅ»øöĻąĶ¬µÄ2LĆܱÕČŻĘ÷ÖŠ¼ÓČė2mol I2£Øg£©”¢4mol H2£Øg£©·¢Éś·“Ó¦£¬“ļĘ½ŗāŹ±£¬HIµÄĢå»ż·ÖŹżŹĒ £°.£¶£ØĢī“óÓŚ”¢µČÓŚ”¢Š”ÓŚ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | +2£¬+2 | B£® | +2£¬+3 | C£® | +2£¬+4 | D£® | +3£¬+3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗÓ±±Ź”ø߶žÉĻµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŅŃÖŖ·“Ó¦nA£Øg£© An£Øg£©µÄĘ½ŗā³£ŹżĪŖK£¬ŌņÓŠ1/2An £Øg£©
An£Øg£©µÄĘ½ŗā³£ŹżĪŖK£¬ŌņÓŠ1/2An £Øg£©  1/2nA£Øg£©µÄĘ½ŗā³£Źż£Ø £©
1/2nA£Øg£©µÄĘ½ŗā³£Źż£Ø £©
A£®K B£®K”„1/2 C£®K2 D£®K”„2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğŗÓ±±Ź”ĢĘɽŹŠø߶žÉĻ10ŌĀŌĀæ¼»ÆѧŹŌ¾ķ £Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŅŃÖŖĻĀĮŠŹż¾Ż£ŗ
4Al(s)£«3O2(g)£½2Al2O3(s) ”÷H£½£3350kJ”¤mol£1
2Fe(s)£«O2(g)£½2FeO(s) ”÷H£½£544kJ”¤mol£1
Ōņ2Al(s) +3FeO(s)£½Al2O3(s) + 3Fe(s)µÄ”÷HŹĒ£Ø £©
A£®£859 kJ”¤mol£1 B£®£«1403 kJ”¤mol£1 C£®£1718 kJ”¤mol£1 D£®£2806kJ”¤mol£1
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com