
»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖÖ÷µÄæĘѧ£¬»ÆѧŹµŃéŹĒѧĻ°Ģ½¾æĪļÖŹŠŌÖŹµÄ»ł±¾·½·ØÖ®Ņ»”£
£Ø1£©ĻĀĮŠÓŠ¹ŲŠšŹöÕżČ·µÄŹĒ__________£ØĢīŠ“ŠņŗÅ£©
a£®Ź¹ÓĆĶŠÅĢĢģĘ½µÄµŚŅ»²½²Ł×÷ŹĒ½«ÓĪĀėŅĘÖĮ±ź³ßĮćæĢ¶Č“¦
b£®¹żĀĖ²Ł×÷¹ż³ĢÖŠ£¬ĪŖ¼Óæģ¹żĀĖĖŁ¶ČæÉÓĆ²£Į§°ō¶ŌĀ©¶·ÖŠµÄČÜŅŗ½ųŠŠ½Į°č
c£®ÓĆÅØĮņĖįÅäÖĘĻ”ČÜŅŗŹ±£¬ŌŚĮæĶ²ÖŠŗāĻ”ŗóŅŖĄäČ“ÖĮŹŅĪĀŌŁ×ŖŅʵ½ČŻĮæĘæÖŠ
d£®ÓĆČŻĮæĘæÅäÖĘČÜŅŗŹ±£¬¶ØČŻŗóŅ”ŌČŅŗĆęĻĀ½µ£¬ŌŁ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß“¦£¬ĖłµĆČÜŅŗÅضČĘ«µĶ
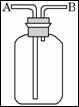 £Ø2£©ĻÖÓŠĮłÖÖĘųĢå£ŗH2”¢O2”¢NH3”¢SO2”¢NO2”¢NO”£æÉŅŌĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹÕ¼Æ”£
£Ø2£©ĻÖÓŠĮłÖÖĘųĢå£ŗH2”¢O2”¢NH3”¢SO2”¢NO2”¢NO”£æÉŅŌĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹÕ¼Æ”£
¢ŁČōĘųĢå“ÓBæŚ½ųČė£¬æÉŹÕ¼ÆµÄĘųĢåŹĒ_______________£»
¢ŚČōŌŚÉÕĘæ֊עĀśĖ®£¬ŌņĘųĢåÓ¦øĆ“Ó___£ØĢīŠ“”°A”±»ņ”°B”±£©æŚ½ųČė£¬æÉŅŌŹÕ¼ÆµÄĘųĢåŹĒ_____”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓɱłĶ£ØmCu2O·nFeS£©Ņ±Į¶µĆµ½“ÖĶ£¬ŌŁŅŌ“ÖĶĪŖŌĮĻÖĘ“æĶµÄĮ÷³ĢČēĻĀ£ŗ
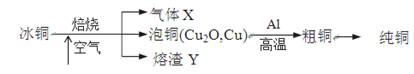
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĘųĢåXŹĒ ”£
£Ø2£©Ä³ŃŠ¾æŠŌѧĻ°Š”×éÓĆČŪŌüYÓėCO·“Ó¦Ą“ÖĘČ”Fe”£
¢ŁĒė°“ĘųĮ÷ÓÉ×óµ½ÓŅµÄ·½ĻņĮ¬½ÓĻĀĮŠø÷×°ÖĆ£¬Ė³ŠņĪŖA”ś________”£
¢Ś×°ÖĆCµÄ×÷ÓĆŹĒ________________”£
¢ŪŌŚµćČ¼D“¦µÄ¾Ę¾«µĘĒ°£¬Ó¦½ųŠŠµÄ²Ł×÷ŹĒ__________________”£

£Ø3£©ČŪŌüYÖŠĢśŌŖĖŲµÄ¼ŪĢ¬ÓŠ+2¼ŪŗĶ+3¼Ū£¬øł¾ŻĻŽŃ”ŹŌ¼Į£¬Éč¼ĘŹµŃé·½°øŃéÖ¤ČŪŌüYÖŠÓŠ+2¼ŪĢśŌŖĖŲ“ęŌŚ£¬Š“³öÓŠ¹ŲŹµŃé²Ł×÷”¢Ō¤ĘŚĻÖĻóÓė½įĀŪ”£
ĻŽŃ”ŹŌ¼Į£ŗ 3 mol·L-1H2SO4”¢6 mol·L-1HNO3”¢3£„ H2O2”¢0.01 mol·L-1KMnO4”¢20£„ KSCN”£ 
 ӣ
ӣ
£Ø4£©Š“³öÅŻĶŅ±Į¶“ÖĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©øł¾ŻĻŽŃ”²ÄĮĻ»³öÓĆ“ÖĶĢįĮ¶“æĶµÄ×°ÖĆĶ¼£¬²¢½ųŠŠ±ŲŅŖµÄ±ź×¢”£
ĻŽŃ”²ÄĮĻ£ŗFeSO4(aq)”¢CuSO4(aq)”¢“ÖĢś”¢“æĢś”¢“ÖĶ”¢“æĶ”¢ÉÕ±”¢Ö±Į÷µēŌ“”¢µ¼Ļß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŃĢŃĢĪķÖŠŗ¬ÓŠCO”¢CO2”¢SO2”¢H2OµČĘųĢ唣ÓĆa.ĪŽĖ®ĮņĖįĶ”¢b.³Ī ĒåŹÆ»ŅĖ®”¢c.ŗģČČŃõ
ĒåŹÆ»ŅĖ®”¢c.ŗģČČŃõ
»ÆĶ”¢d.ÉśŹÆ»Ņ”¢e.Ę·ŗģČÜŅŗµČæɽ«ĘäŅ»Ņ»¼ģ³ö£¬¼ģ³öµÄÕżČ·Ė³ŠņŹĒ
A£®»ģŗĻĘų”śa”śe”śe”śb”śd”śc B£®»ģŗĻĘų”śc”śd”śe”śe”śa
C£®»ģŗĻĘų”śa”śe”śb”śa”śd”śc D£®»ģŗĻĘų”śb”śe”śa”śd”śc
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ: £Ø £©
¢Ł³żČ„¶žŃõ»ÆĮņÖŠµÄÉŁĮæČżŃõ»ÆĮņæÉÓĆ98%µÄÅØĮņĖį
¢ŚŹ¢äåµÄŹŌ¼ĮĘæĄļ¼ÓÉŁĮæĖ®ŅŌ¼õÉŁäåµÄ»Ó·¢
¢Ū¼Ų”¢ÄĘ”¢°×Į׶¼Ó¦±£“ęŌŚĖ®ÖŠ
¢Ü×öŃęÉ«·“Ó¦ŹµŃ鏱ĖłÓĆ²¬Ė棬Ćæ“ĪÓĆĶźŗóÓĆĻ”ĮņĖįĻ“µÓŗóŌŁŹ¹ÓĆ
A£®¢Ł¢Ś B £®¢Ś¢Ü C£®¢Ł¢Ū
£®¢Ś¢Ü C£®¢Ł¢Ū  D£®¢Ł¢Ś¢Ü
D£®¢Ł¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲŹŌ¼ĮµÄ±£“ę·½·Ø£¬“ķĪóµÄŹĒ
A£®Ēā·śĖį±£“ęŌŚĪŽÉ«²£Į§ŹŌ¼ĮĘæÖŠ
B£®ĒāŃõ»ÆÄĘČÜŅŗ±£“ęŌŚ¾ßĻšĘ¤ČūµÄ²£Į§ŹŌ¼ĮĘæÖŠ
C£®ÉŁĮæµÄÄʱ£“ęŌŚĆŗÓĶÖŠ
D£®ŠĀÖʵÄĀČĖ®Ķس£±£“ęŌŚ×ŲÉ«²£Į§ŹŌ¼ĮĘæÖŠ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ
A£®ĀĮČÜÓŚĻ”ĮņĖį£ŗAl+2H+=Al3++H2”ü£¬
B£®Ģś·ŪČÜÓŚĻ”ĮņĖį£ŗFe+2H+=Fe2++H2”ü£¬
C£®“óĄķŹÆČÜÓŚ“×Ėį£ŗCaCO3+2H+=Ca2++CO2”ü+H2O£¬
D£®ĒāŃõ»Æ±µČÜŅŗŗĶĻ”ĮņĖį»ģŗĻ£ŗBa2++SO 42£=BaSO4”ż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŹĒÖʱøCuOµÄĮ÷³ĢĶ¼£ŗ
¹¤ŅµCuSO4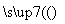
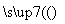 CuSO4ČÜŅŗ
CuSO4ČÜŅŗ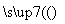 CuSO4·5H2O”Ŗ”ś”””Ŗ”śCuO
CuSO4·5H2O”Ŗ”ś”””Ŗ”śCuO
£Ø1£©²½Öč¢ńµÄÄæµÄŹĒ³żČ„²»ČÜŠŌŌÓÖŹ”£²Ł×÷ŹĒ________________________________”£
£Ø2£©²½Öč¢ņµÄÄæµÄŹĒ³żĢś”£²Ł×÷ŹĒ£ŗµĪ¼ÓH2O2ČÜŅŗ£¬ÉŌ¼ÓČČ£»µ±Fe2£«×Ŗ»ÆĶźČ«ŗó£¬ĀżĀż¼ÓČėCu2(OH)2CO3·ŪÄ©£¬½Į°č£¬ŅŌæŲÖĘČÜŅŗpH£½3.5£»¼ÓČČÖó·ŠŅ»¶ĪŹ±¼ä£¬¹żĀĖ£¬ÓĆĻ”ĮņĖįĖį»ÆĀĖŅŗÖĮpH£½1”£æŲÖĘČÜŅŗpH£½3.5µÄŌŅņŹĒ________________________________________________”£
£Ø3£©²½Öč¢óµÄÄæµÄŹĒµĆµ½CuSO4·5H2O¾§Ģ唣²Ł×÷ŹĒ_____________________£¬¹żĀĖ£¬Ė®Ō”¼ÓČČŗęøÉ”£Ė®Ō”¼ÓČȵÄĢŲµćŹĒ___ ________________________________”£
________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠŅ»ĘæĒ©ÉĻ×¢Ć÷ĪŖĘĻĢŃĢĒĖįŃĪ£ØÄĘ”¢Ć¾”¢øĘ”¢Ģś£©µÄø“ŗĻÖĘ¼Į£¬Ä³Ķ¬Ń§ĪŖĮĖČ·ČĻĘä³É
·Ö£¬Č”²æ·ÖÖĘ¼Į×÷ĪŖŹŌŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
ŅŃÖŖ£ŗæŲÖĘČÜŅŗpH£½4Ź±£¬Fe(OH)3³ĮµķĶźČ«£¬Ca2£«”¢Mg2£«²»³Įµķ”£
øĆĶ¬Ń§µĆ³öµÄ½įĀŪÕżČ·µÄŹĒ
A£®øł¾ŻĻÖĻó1æÉĶĘ³öøĆŹŌŅŗÖŠŗ¬ÓŠNa£«
B£®øł¾ŻĻÖĻó2æÉĶĘ³öøĆŹŌŅŗÖŠ²¢²»ŗ¬ÓŠĘĻĢŃĢĒĖįøł
C£®øł¾ŻĻÖĻó3ŗĶ4æÉĶĘ³öøĆŹŌŅŗÖŠŗ¬ÓŠCa2£«£¬µ«Ć»ÓŠMg2£«
D£®øł¾ŻĻÖĻó5æÉĶĘ³öøĆŹŌŅŗÖŠŅ»¶Øŗ¬ÓŠFe2£«
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com