
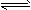 H++Cl-+HClO����������
H++Cl-+HClO����������

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
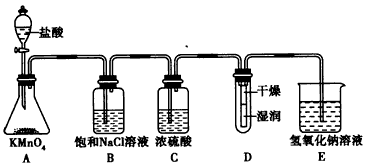
��12�֣���ҵ�Ͻ��������������ͨ�뵽���ʵ���Ũ��Ϊ0.375mol/L��NaOH��Һ�еõ�Ưˮ��ijͬѧ��ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��
(1)����470mL���ʵ���Ũ��Ϊ0.375 mol/L��NaOH��Һʱ����Ҫ�õ��IJ����������ձ�������������ͷ�ιܡ���Ͳ�� ��
(2)Ũ�����������______________________��
(3)װ��E�з�����Ӧ�����ӷ���ʽΪ______________________________________��
(4)װ��B�б���NaCl��Һ�����ڳ�ȥCl2�е�HCl���壬��֪������ˮ�ķ�Ӧ��һ�����淴Ӧ��������ñ���NaCl��Һ��ȥCl2��HCl�����ԭ��(�����ӷ���ʽ��ʾ�������Ҫ������˵��)
��
(5)ʵ��ʱװ��D��ʪ��ĺ�ɫֽ����ɫ�����ﲿ��û����ɫ������һ��ʱ���ֽ��ȫ����ɫ����ͬѧ��ΪCl2���ܶȱȿ����������Թ��²�Cl2��Ũ�ȴ����Թ��ϲ�Cl2��Ũ�������µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________
_________________________________________��
(6)����Ƶ���Ԫ�غ���Ϊ10%��Ưˮ��Һ35.5g����ô���к�NaClO�����ʵ���Ϊ____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��ҵ�Ͻ��������������ͨ�뵽���ʵ���Ũ��Ϊ0.375 mol��L��1NaOH��Һ�еõ�Ư��ˮ��ijͬѧ����ʵ����̽��Cl2���ʲ�ģ���Ʊ�Ư��ˮ����ͼ�Dz���ʵ��װ�á���֪KMnO4��������Һ��Ӧ������ȡCl2��
��
��1���������ʵ���Ũ��Ϊ0.375 mol��L��1NaOH��Һ100mLʱ, ��������ƽ��ȡ�����NaOH����Ϊ�������� ��
��2��Ũ��������������� ������
��3��װ��E�з�����ѧ��Ӧ����ʽΪ���� ���� ��
��4��װ��B�б���NaCl��Һ�������� ��
��5��ʵ��ʱװ��D��ʪ��ĺ�ɫֽ����ɫ�����ﲿ��û����ɫ������һ��ʱ���ֽ��ȫ����ɫ����ͬѧ��ΪCl2���ܶȱȿ����������Թ��²�Cl2��Ũ�ȴ����Թ��ϲ�Cl2��Ũ�������µġ����жϸý����Ƿ��������������������ʵ�������ԭ��������
�� ������Ľ����������Ʒ������� ����������Ϊ����������ʿɲ����𣩡�
��6����Ԫ�غ���Ϊ10%��Ư��ˮ��Һ355g�����к�NaClO�����ʵ���Ϊ��������mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ�������谲һ�и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
(15��)��ҵ�Ͻ��������������ͨ�뵽���ʵ���Ũ��Ϊ0.375 mol��L��1NaOH��Һ�еõ�Ư��ˮ��ijͬѧ����ʵ����̽��Cl2���ʲ�ģ���Ʊ�Ư��ˮ����ͼ�Dz���ʵ��װ�á���֪KMnO4��������Һ��Ӧ������ȡCl2��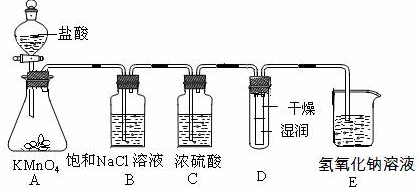 ��
��
��1���������ʵ���Ũ��Ϊ0.375 mol��L��1NaOH��Һ100mLʱ, ��������ƽ��ȡ�����NaOH����Ϊ�������� ��
��2��Ũ��������������� �� ����
��3��װ��E�з�����ѧ��Ӧ����ʽΪ���� ���� ��
��4��װ��B�б���NaCl��Һ�������� ��
��5��ʵ��ʱװ��D��ʪ��ĺ�ɫֽ����ɫ�����ﲿ��û����ɫ������һ��ʱ���ֽ��ȫ����ɫ����ͬѧ��ΪCl2���ܶȱȿ����������Թ��²�Cl2��Ũ�ȴ����Թ��ϲ�Cl2��Ũ�������µġ����жϸý����Ƿ��������������������ʵ�������ԭ��������
�� ������Ľ����������Ʒ������� ���� ������Ϊ����������ʿɲ����𣩡�
��6����Ԫ�غ���Ϊ10%��Ư��ˮ��Һ355g�����к�NaClO�����ʵ���Ϊ�������� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��12�֣���ҵ�Ͻ��������������ͨ�뵽���ʵ���Ũ��Ϊ0.375mol/L��NaOH��Һ�еõ�Ưˮ��ijͬѧ��ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��

(1)����470mL���ʵ���Ũ��Ϊ0.375 mol/L��NaOH��Һʱ����Ҫ�õ��IJ����������ձ�������������ͷ�ιܡ���Ͳ�� ��
(2)Ũ�����������______________________��
(3)װ��E�з�����Ӧ�����ӷ���ʽΪ______________________________________��
(4)װ��B�б���NaCl��Һ�����ڳ�ȥCl2�е�HCl���壬��֪������ˮ�ķ�Ӧ��һ�����淴Ӧ��������ñ���NaCl��Һ��ȥCl2��HCl�����ԭ��(�����ӷ���ʽ��ʾ�������Ҫ������˵��)
��
(5)ʵ��ʱװ��D��ʪ��ĺ�ɫֽ����ɫ�����ﲿ��û����ɫ������һ��ʱ���ֽ��ȫ����ɫ����ͬѧ��ΪCl2���ܶȱȿ����������Թ��²�Cl2��Ũ�ȴ����Թ��ϲ�Cl2��Ũ�������µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________
_________________________________________��
(6)����Ƶ���Ԫ�غ���Ϊ10%��Ưˮ��Һ35.5g����ô���к�NaClO�����ʵ���Ϊ____mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com