
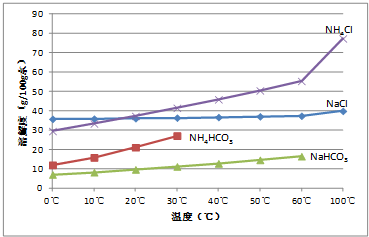
| c��(V2-2V1)��10-3mol��84g/mol |
| W g |
| 8.4c(V2-2V1) |
| W |
| 8.4c(V2-2V1) |
| W |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ�鲽�� | Ԥ������ͽ��� | |
| �� | ȡ��������Ư�����Թ��У� ��������1mol/L�����ܽ���ٽ����������嵼�뵽����ʯ��ˮ�� ��������1mol/L�����ܽ���ٽ����������嵼�뵽����ʯ��ˮ�� |
��1��������ʯ��ˮδ�����ǣ������1��������2��������ʯ��ˮ����ǣ������2�����3���� ��1��������ʯ��ˮδ�����ǣ������1��������2��������ʯ��ˮ����ǣ������2�����3���� |
| �� | ����ٷ�Ӧ����Թܵ���1��2��Ʒ����Һ���� ����ٷ�Ӧ����Թܵ���1��2��Ʒ����Һ���� |
��1����Ʒ����ɫ�������3��������2����Ʒ�첻��ɫ�������2���� ��1����Ʒ����ɫ�������3��������2����Ʒ�첻��ɫ�������2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | ����/g | |
| ��1�� | 196.30 | |
| ��2�� | 196.15 | |
| ��ƿʮˮʮ���� | ��3�� | 196.05 |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
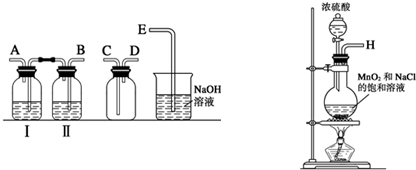
| ���� | ���� |
| ȡ4gƯ�۾����壬����100mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
 |
����Һ���Ϸ�������״�� �����Ժ��ֻ��ǣ���Һ��Ϊ����ɫ �����Ժ���������ɫ����������ɫ��ȥ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com