
���� ��ɫ����������NaOH��Һ��ӦΪAl��OH��3��˵�����к���Al��H����Ԫ�أ�n��H2��=$\frac{6.72L}{22.4L/mol}$=0.3mol��
��m��H��=0.3mol��2��1g/mol=0.6g����6.00g�����m��Al��=6.00g-0.6g=5.4g��n��Al��=$\frac{5.4g}{27g/mol}$=0.2mol��
����n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g•L-1����������ԭ������Ϊ1.25g•L-1��22.4L=28��ӦΪN2������ΪNH3����϶�Ӧ�������Լ���ĿҪ��ɽ����⣮
��� �⣺��ɫ����������NaOH��Һ��ӦΪAl��OH��3��˵�����к���Al��H����Ԫ�أ�n��H2��=$\frac{6.72L}{22.4L/mol}$=0.3mol����m��H��=0.3mol��2��1g/mol=0.6g����6.00g���к���m��Al��=6.00g-0.6g=5.4g��n��Al��=$\frac{5.4g}{27g/mol}$=0.2mol������n��Al����n��H��=0.2mol��0.6mol=1��3����Ļ�ѧʽΪAlH3�����ڱ�״���µ��ܶ�Ϊ1.25g•L-1����������ԭ������Ϊ1.25g•L-1��22.4L=28��ӦΪN2������ΪNH3��
��1�������Ϸ�����֪��ΪAlH3���ʴ�Ϊ��AlH3��
��2��AlH3��ˮ����������ԭ��Ӧ����Ӧ�ķ���ʽΪAlH3+3H2O=Al��OH��3��+3H2����
�ʴ�Ϊ��AlH3+3H2O=Al��OH��3��+3H2����
��3��þ���ڵ�����ȼ�����ɵ���þ����ѧʽΪMg3N2���ʴ�Ϊ��Mg3N2��
��4��NH3�ڼ�����������CuO��Ӧ������Cu������N2����Ӧ�ķ���ʽΪ3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O���ʴ�Ϊ��3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$N2+3Cu+3H2O��
��5��Ҫ�жϲ������Ƿ���CuO���ɼ���ϡ���������Һ�Ƿ������������ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O��
�ʴ�Ϊ��ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬����Al���仯����ת��Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע�������ط�Ӧ�������Լ������жϼ�����������Ϊ����ͻ�ƿڣ���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 37.6% | B�� | 36.8% | C�� | 25.6% | D�� | 51.2% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ǽ���ԭ�Ӽ��Թ��ۼ���ϵ����ʶ��ǹ��ۻ����� | |
| B�� | �ɲ�ͬԭ�����γɵĴ�����һ���ǻ����� | |
| C�� | ���н���Ԫ�ص����Ӳ�һ���������� | |
| D�� | ���ӻ�����һ���ܵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2��3 | B�� | 2��5 | C�� | 1��2 | D�� | 1��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
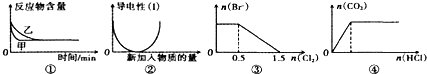
| A�� | ͼ�ٱ���ѹǿ�ɶ��淴Ӧ2A��g��+2B��g��?3C��g��+D��g����Ӱ�죬�ҵ�ѹǿ�� | |
| B�� | ͼ�ڱ�ʾ��������Һ��ͨ�백���������Ĺ��̣���Һ�����Ա仯 | |
| C�� | ͼ�۱�ʾ��1L1mol/LFeBr2��Һ��ͨ��Cl2ʱBr-�����ı仯 | |
| D�� | ͼ�ܱ�ʾһ��Ũ��Na2CO3��Һ����εμ�����������CO2�����ʵ����Ĺ�ϵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 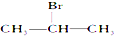 | B�� | 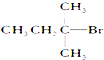 | C�� | 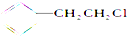 | D�� | CH3CH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
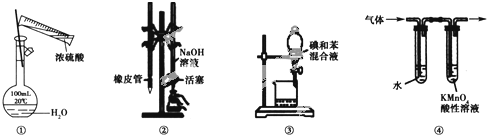
| A�� | ͼ����ʾװ�ý���Ũ����ϡ������ϡ�����ʵ�� | |
| B�� | ͼ����ʾװ�ý�������֪Ũ��ΪNaOH��Һ�ⶨ����Ũ�ȵ�ʵ�� | |
| C�� | ͼ����ʾװ�ý����ñ���ȡ��ˮ�е��ʵ�飬���ѵ�ı���Һ��©���¿ڵ��� | |
| D�� | ͼ����ʾװ�ÿɼ��������鷢����ȥ��Ӧ�õ��������к�����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=l����Һ�У�Ca2+��Fe2+��NO3- | |
| B�� | $\frac{Kw}{c��O{H}^{-}��}$=10-2����Һ�У�Na+��NH4+��Cl- | |
| C�� | c��Fe3+��=0.1 mol��L-1����Һ�У�K+��SCN-��SO42- | |
| D�� | ��ˮ�����c��H+��=1��10-14 mol��L-1����Һ�У�Na+��AlO2-��CO32- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com