
| 71g+32g |
| 2mol |


 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | K+���� ��Na+���� Cu2+���� Al3+ |
| ������ | SO42-���� HCO3-�� ��NO3-�� ��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ԭ�ӵ�2p�������ԭ�ӵ�1s��� |
| B����ԭ�ӵ�2p�������ԭ�ӵ�2p��� |
| C����ԭ�ӵ�3p�������ԭ�ӵ�1s��� |
| D����ԭ�ӵ�2p�������ԭ�ӵ�3p��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��24 gþ��ԭ������������ΪNA |
| B��1 L 0.1 mol?L-1������Һ��H+��Ϊ0.1NA |
| C��1 mol�����������������Ϊ10NA |
| D����״���£�22.4 L�Ҵ��ķ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol ������6nA��C-H�� |
| B����״���£�22.4L��ˮ����nA��NH3���� |
| C��18gH2O����10nA������ |
| D��56g��ƬͶ������ŨH2SO4������nA��SO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
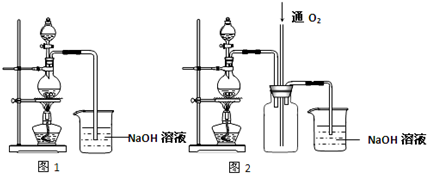
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������˷�̪��Һ |
| B����������NaOH��Һ |
| C����������ʯ����Һ |
| D�����������κ��Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����̬̼ԭ�ӵļ۵����Ų�ͼ��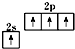 |
| B��HClO�Ľṹʽ��H-Cl-O |
| C����̬26Fe�ļ۵����Ų�ʽ��3d64s2 |
D����̬ͭԭ�ӵļ۵����Ų�ͼ��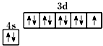 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com