
�±������ڱ��е�һ���֣�����A��I�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺
|
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H |
(1)����Ԫ�أ���ѧ��������õ��� ��ֻ�и��۶������۵��� ��
��������ǿ�ĵ����� ����ԭ����ǿ�ĵ����� ��
(2)����������ˮ���������ǿ���� ��������ǿ���� �������Ե��� ��
(3)A�ֱ���D��E��F��G��H�γɵĻ������У����ȶ��� ��
(4)��B��C��D��E��F��G��H�У�ԭ�Ӱ뾶������ ��
(5��A��E�γɻ�����Ļ�ѧʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | |||
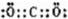
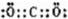
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����а�У������һ���£����л�ѧ�Ծ��������棩 ���ͣ������
| ������ | IA | |||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | |||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com