
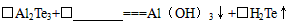

 ĆūÅĘѧŠ£·Ö²ćÖÜÖܲāĻµĮŠ“š°ø
ĆūÅĘѧŠ£·Ö²ćÖÜÖܲāĻµĮŠ“š°ø »ĘøŌŗ£µķČ«³ĢÅąÓŲāŹŌ¾ķĻµĮŠ“š°ø
»ĘøŌŗ£µķČ«³ĢÅąÓŲāŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ŌŖĖŲ“śŗÅ | Ļą¹ŲŠÅĻ¢ |
| T | TµÄµ„ÖŹÄÜÓėĄäĖ®¾ēĮŅ·“Ó¦£¬ĖłµĆĒæ¼īŠŌČÜŅŗÖŠŗ¬ÓŠĮ½ÖÖµē×ÓŹżĻąĶ¬µÄŅõ”¢ŃōĄė×Ó |
| X | XµÄŌ×Ó×īĶā²ćµē×ÓŹżŹĒĘäÄŚ²ćµē×ÓŹżµÄČż±¶ |
| Y | ŌŚµŚČżÖÜĘŚ½šŹōŌŖĖŲÖŠ£¬YµÄ¼ņµ„Ąė×Ó°ė¾¶×īŠ” |
| Z | T”¢X”¢Z×é³ÉµÄ36µē×ӵĻÆŗĻĪļAŹĒ¼ŅÓĆĻū¶¾¼ĮµÄÖ÷ŅŖ³É·Ö |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗÓ±±Ź”ŗāĖ®ÖŠŃ§ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø11·Ö£©T”¢X”¢Y”¢Z”¢Q”¢R”¢WĪŖÖÜĘŚ±ķĒ°ĖÄÖÜĘŚŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪµŻŌö£¬Ęä֊ijŠ©ŌŖĖŲµÄĻą¹ŲŠÅĻ¢ČēĻĀ±ķ£ŗ
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢ |
| T | TŌ×ÓĖł“¦µÄÖÜĘŚŹż”¢×åŠņŹż·Ö±šÓėĘäŌ×ÓŠņŹżĻąµČ |
| X | XµÄ»łĢ¬Ō×ÓÖŠµē×ÓÕ¼¾ŻČżÖÖÄÜĮæ²»Ķ¬µÄŌ×Ó¹ģµĄ£¬ĒŅĆæÖÖ¹ģµĄÖŠµÄµē×ÓŹżĻąĶ¬ |
| Z | ZµÄ»łĢ¬Ō×Ó¼Ūµē×ÓÅŲ¼ĪŖ |
| Q | ŌŚøĆŌŖĖŲĖłŌŚÖÜĘŚÖŠ£¬QµÄ»łĢ¬Ō×ӵĵŚŅ»µēĄėÄÜ×īŠ” |
| R | 3pÄܼ¶ÉĻÓŠ1øöµē×Ó |
| W | WµÄŅ»ÖÖŗĖĖŲµÄÖŹĮæŹżĪŖ65£¬ÖŠ×ÓŹżĪŖ36 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗÓ±±Ź”ø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĶʶĻĢā
£Ø11·Ö£©T”¢X”¢Y”¢Z”¢Q”¢R”¢WĪŖÖÜĘŚ±ķĒ°ĖÄÖÜĘŚŌŖĖŲ£¬Ō×ÓŠņŹżŅĄ“ĪµŻŌö£¬Ęä֊ijŠ©ŌŖĖŲµÄĻą¹ŲŠÅĻ¢ČēĻĀ±ķ£ŗ
|
ŌŖĖŲ |
Ļą¹ŲŠÅĻ¢ |
|
T |
TŌ×ÓĖł“¦µÄÖÜĘŚŹż”¢×åŠņŹż·Ö±šÓėĘäŌ×ÓŠņŹżĻąµČ |
|
X |
XµÄ»łĢ¬Ō×ÓÖŠµē×ÓÕ¼¾ŻČżÖÖÄÜĮæ²»Ķ¬µÄŌ×Ó¹ģµĄ£¬ĒŅĆæÖÖ¹ģµĄÖŠµÄµē×ÓŹżĻąĶ¬ |
|
Z |
ZµÄ»łĢ¬Ō×Ó¼Ūµē×ÓÅŲ¼ĪŖ |
|
Q |
ŌŚøĆŌŖĖŲĖłŌŚÖÜĘŚÖŠ£¬QµÄ»łĢ¬Ō×ӵĵŚŅ»µēĄėÄÜ×īŠ” |
|
R |
3pÄܼ¶ÉĻÓŠ1øöµē×Ó |
|
W |
WµÄŅ»ÖÖŗĖĖŲµÄÖŹĮæŹżĪŖ65£¬ÖŠ×ÓŹżĪŖ36 |
£Ø1£©X”¢Y”¢QČżÖÖŌŖĖŲµÄµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņŹĒ £ØÓĆŌŖĖŲ·ūŗűķŹ¾£©”£
£Ø2£©XÓėYŌ×Ó½įŗĻŠĪ³ÉµÄX3Y4¾§Ģ壬¾§Ģå½į¹¹Óė½šøÕŹÆĄąĖĘ£¬ŌņX3Y4¾§ĢåµÄČŪµć±Č½šøÕŹÆŅŖ £ØĢī”°øß”±”¢”°µĶ”±£©”£
£Ø3£©W2+µÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ ”£ŌŖĖŲWÓėČĖĢå·ÖĆŚĪļÖŠµÄŃĪĖįŅŌ¼°æÕĘų·“Ó¦æÉÉś³É³¬ŃõĖį£ŗW£«HCl£«O2£½WCl£«HO2£¬HO2 £Ø³¬ŃõĖį£©²»½öŹĒŅ»ÖÖČõĖį¶ųĒŅŅ²ŹĒŅ»ÖÖ×ŌÓÉ»ł£¬¾ßÓŠ¼«øߵĻīŠŌ”£ĻĀĮŠĖµ·Ø»ņ±ķŹ¾“ķĪóµÄŹĒ
A£®Ńõ»Æ¼ĮŹĒO2 B£®HO2ŌŚ¼īÖŠ²»ÄÜĪČ¶Ø“ęŌŚ
C£®Ńõ»Æ²śĪļŹĒHO2 D£®1 molW²Ī¼Ó·“Ó¦ÓŠ1 molµē×Ó·¢Éś×ŖŅĘ
£Ø4£©X”¢Y”¢Z·Ö±šÓėĒāŌŖĖŲæÉŅŌ¹¹³ÉA”¢B”¢C”¢DµČ¶ąÖÖĮ£×Ó”£ĘäÖŠA”¢B”¢C¾łĪŖ10µē×ÓĪ¢Į££¬DĪŖ18µē×ÓĪ¢Į£”£AĪŖ5Ō×ÓŗĖµÄ+1¼ŪŃōĄė×Ó£¬ŌņA+µÄÖŠŠÄŌ×ÓŌӻƷ½Ź½ĪŖ_______. BĪŖ4Ō×ÓŗĖµÄ+1¼ŪŃōĄė×Ó£¬ŌņB+µē×ÓŹ½ĪŖ___________”£CĪŖ4øöŌ×ÓŗĖ¹¹³ÉµÄ·Ö×Ó£¬ŌņÓėC»„ĪŖµČµē×ÓĢåµÄ·Ö×ÓæÉŅŌŹĒ_______£ØŠ“½į¹¹Ź½£©”£D·Ö×ÓÖŠĮ½ŌŖĖŲµÄŌ×ÓøöŹżÖ®±ČĪŖ1:1£¬ŌņDĪŖ £ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©·Ö×Ó”£Ä³Ė«Ō×Óµ„ÖŹ·Ö×ÓEŅ²ĪŖ18µē×ÓĪ¢Į££¬EÓėĖ®µÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________”£
£Ø5£©ŅŃÖŖ25”ę”¢101 kPaĢõ¼žĻĀ£ŗ
4R£Øs£©+3Z2£Øg£© 2R2Z3£Øs£© ”÷H=-2835£®9 kJ£Æmol
4R£Øs£©+2Z3£Øg£© 2R2Z3£Øs£© ”÷H=-3119£®1 kJ£Æmol
Ōņ16g Z2£Øg£©ĶźČ«×Ŗ»ÆĪŖZ3£Øg£©µÄ”÷H= £®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
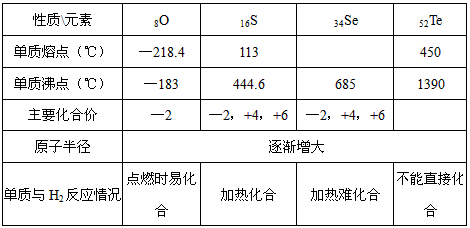
ĻĀ±ķŹĒŃõ×åŌŖĖŲµÄĻą¹ŲŠŌÖŹ
| ŠŌÖŹ\ŌŖĖŲ | 8O | 16S | 34Se | 52Te |
| µ„ÖŹČŪµć£Ø”ę£© | ØD218.4 | 113 | 450 | |
| µ„ÖŹ·Šµć£Ø”ę£© | ØD183 | 444.6 | 685 | 1390 |
| Ö÷ŅŖ»ÆŗĻ¼Ū | ØD2 | ØD2£¬+4£¬+6 | ØD2£¬+4£¬+6 | |
| Ō×Ó°ė¾¶ | Öš½„Ōö“ó | |||
| µ„ÖŹÓėH2·“Ó¦Ēéæö | µćČ¼Ź±Ņ×»ÆŗĻ | ¼ÓČČ»ÆŗĻ | ¼ÓČČÄŃ»ÆŗĻ | ²»ÄÜÖ±½Ó»ÆŗĻ |
Ēėøł¾Ż±ķ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĪųµÄČŪµć·¶Ī§æÉÄÜŹĒ
£Ø2£©ĒāĪųĖįÓŠ½ĻĒæµÄ £ØĢī”°Ńõ»ÆŠŌ”±»ņ”°»¹ŌŠŌ”±£©£¬Ņņ“Ė·ÅŌŚæÕĘųÖŠ³¤ĘŚ±£“ęŅ×±äÖŹ£¬ĘäæÉÄÜ·¢ÉśŌŚ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
£Ø3£©¹¤ŅµÉĻAl2Te3æÉÓĆĄ“ÖʱøH2Te£¬Ķź³ÉĻĀĮŠ»Æѧ·½³ĢŹ½£ŗ
”õAl2Te3+”õ ===Al£ØOH£©3”ż+”õH2Te”ü
£Ø4£©ŅŃÖŖŌŚ³£ĪĀĻĀ£¬H2ŗĶS·“Ӧɜ³É17gH2S·Å³ö56.1KJµÄČČĮ棬ŹŌŠ“³öĮņ»ÆĒā·Ö½āµÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø5£©ÓŅĶ¼ĖłŹ¾ĪŖŃõ×åŌŖĖŲµ„ÖŹÓėH2·“Ó¦¹ż³ĢÖŠµÄ
ÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬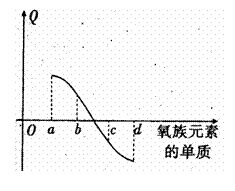 ĘäÖŠa”¢b”¢c”¢d·ÖĮķ±ķŹ¾
ĘäÖŠa”¢b”¢c”¢d·ÖĮķ±ķŹ¾
Ńõ×å֊ijŅ»ŌŖĖŲµÄµ„ÖŹ£¬QĪŖĻąĶ¬ĪļÖŹµÄĮæ
µÄµ„ÖŹÓėH2·“Ó¦·Å³öµÄČČĮ攣Ōņ£ŗ
a“ś±ķ £¬c“ś±ķ
£Ø¾łæɵ„ÖŹĆū³Ę£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com